Translate this page into:
Responsible antibiotic therapy simplified
*Corresponding author: Dr. Abhay K. Shah, Senior Pediatrician and Infectious Diseases Consultant, Director, Children Hospital, 5 Mehta Apartments, Maninagar, Ahmedabad - 380008, Gujarat, India. drabhaykshah@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Shah AK, Shah AA. Responsible antibiotic therapy simplified. Karnataka Paediatr J 2020;35(1):29-38.
Abstract
Antimicrobial resistance is a global problem and is particularly pressing in developing countries where the infectious disease burden is very high. In developing countries, where relatively easy availability and higher consumption of medicines have led to disproportionately higher incidence of inappropriate use of antibiotics and greater levels of resistance compared to developed countries. The bacterial disease burden in India is among the highest in the world; consequently, antibiotics will play a critical role in limiting morbidity and mortality in the country. Improving antibiotic prescribing and use is critical to effectively treat infections, protect patients from harms caused by unnecessary antibiotic use, and combat antibiotic resistance. Responsible antibiotic therapy is one of the most important components of antibiotic stewardship. The current article is an attempt to provide a set of key principles to guide efforts to improve responsible and rational antibiotic use.
Keywords
Antibiotic
Rational therapy
Antimicrobial resistance
INTRODUCTION
Antimicrobial resistance (AMR) is a global problem and is particularly pressing in developing countries where the infectious disease burden is very high. Easy availability and irrational use of antimicrobials and relatively higher use of these molecules are associated with higher levels of resistance in developing countries as compared to developed countries.[1] In India, the infectious disease burden is very high resulting in more rampant use of antimicrobial agents which are often found to be inappropriate and irrational leading to increase in the development of AMR.[2]
What is the need for responsible antibiotic therapy?
Penicillin, the first antibiotic, was discovered by Alexander Fleming the in 1928, and then it was introduced on a large scale for the treatment of bacterial infections in 1945. This marked the beginning of the so-called “golden era” of antibiotics (1945–1962). However, irresponsible use of antibiotics has made so many antibiotics a toothless weapon to fight against many bacterial infections. As a result antibiotic-resistant bacteria are emerging very fast and are becoming more and more difficult to manage, making available antibiotics ineffective. The antibiotic pipeline is becoming dryer and dryer and today, we are left with very few novel antibiotic classes in the pipeline [Figure 1].[3]
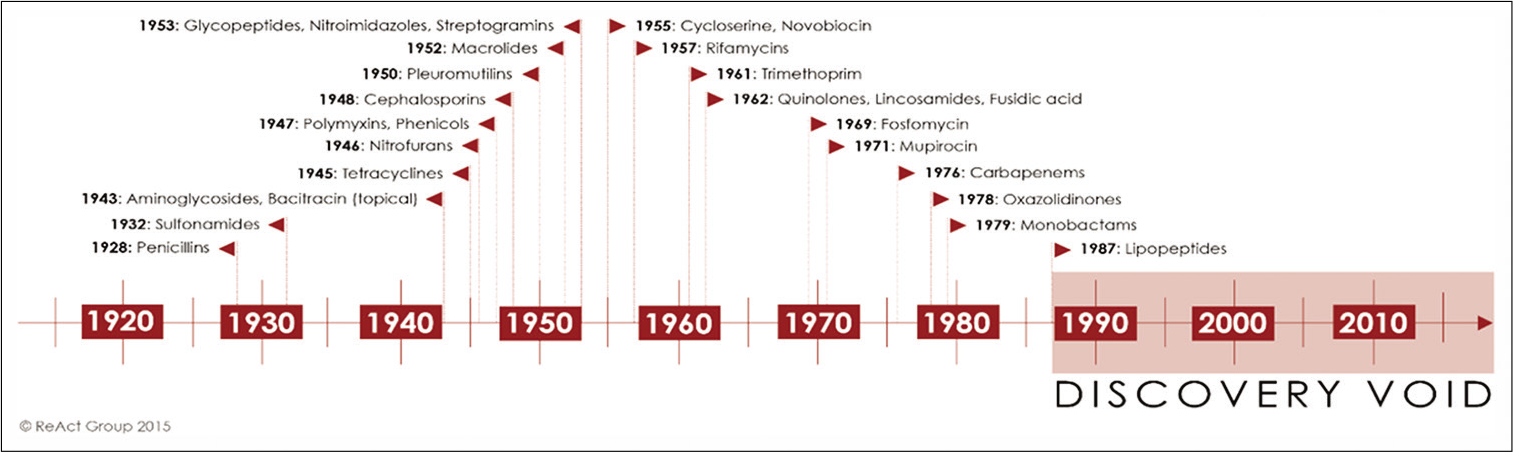
- Time-line of the discovery of different antibiotic classes in clinical use. “The discovery void” refers to the period from 1987 until today, as the last antibiotic class that has been successfully introduced as treatment was discovered in 1987. Adapted from.
It is important to understand that the problem of antibiotic resistance cannot be “solved” by the discovery of one or few new antibiotics. Whenever an antibiotic is used, either in human beings or in animals or plants, whether appropriately or not, the chances of the development and spread of antibiotic-resistant bacteria are increased.[4] The bacterial disease burden in India is among the highest in the world; consequently, antibiotics will play a critical role in limiting morbidity and mortality in the country. It is estimated that by the year 2050, Asia will have 4.7 million deaths that could be directly attributed to AMR.[5] AMR is rampant in India with up to 12–59% of Escherichia coli being extended beta-lactamase (ESBL) producers and up to 30% being carbapenemase producers. Klebsiella pneumonia has emerged over the last few years as a highly resistant pathogen with up to 50% resistance to carbapenems and rapidly increasing resistance to polymyxins. In addition, methicillin resistance in Staphylococcus aureus is seen in up to 30% of S. aureus isolates nationally.[5] There is not much data on high incidence of vancomycin-resistant enterococci in India. However, reduced susceptibility to vancomycin was observed in a study in about 12% of the isolates of Enterococcus faecalis.[6]
It is well documented that antibiotic abuse is one of the major drivers of antibiotic resistance and thus optimizing usage of antibiotics is the need of the hour.
What is irresponsible antibiotic use?
Antibiotics are being prescribed for indications in which their use is not warranted. In India, between 2005 and 2009, the units of antibiotics sold increased by about 40%.[7] Antibiotics are treated as antipyretics and all febrile illnesses are referred as antibiotic deficiency states. This is because of clinicians’ erroneous trust in antibiotics which is further compounded by a wrong belief that they will prevent secondary bacterial infections. Few commonest situations for the antimicrobial misuse are as under:
Fever without focus
Minor skin infections
Nonspecific upper respiratory tract infections such as Rhinitis, and tonsillopharyngitis
Bronchitis
Asthma/Bronchiolitis/WALRI
Gastroenteritis
Non-infectious fever
To prevent secondary bacterial infection in viral illness
To prevent bacterial infection in normal newborns
Asymptomatic bacteriuria and pyuria including in catheterized patients
Microbial colonization and culture contamination.
RESPONSIBLE ANTIBIOTIC THERPY: A SIMPLIFIED APPROACH
When antibiotic is needed?
Definitive: Proven pathogen with or without antibiotic susceptibility result
Empirical: Infection most likely but exact organism and sensitivity not known
Pre-emptive: Infection probable but not proved
Prophylactic for certain conditions only (not discussed in this article).
Definitive antimicrobial therapy
Establishing microbial diagnosis
Antibiotics are ideally needed when there is proven bacterial infection, which can be identified by isolating an organism from sterile body fluids, blood, or tissue from site of infection by culture along with antibiotic susceptibility test. However, in clinical practice this is not always possible because of number of limiting factors.[8]
Tests for detection of organisms have been sent but results are awaited
Affordability and availability
Prior antibiotic use
Slow growing and fastidious organisms
Improper collection and transport of specimen and culture media.
In such cases, non-culture methods should be used to establish diagnosis of bacterial infections. This includes smear examination, antigen detection test, rapid tests, serological tests, and molecular tests such as PCR. Premature initiation of antimicrobial therapy without attempting microbial diagnosis can suppress bacterial growth and preclude the opportunity to establish a microbiological diagnosis, which is at times going to be so critical in the management of certain cases, who may require several weeks to months of directed antimicrobial therapy to achieve cure (e.g., endocarditis, septic arthritis, and meningitis). To optimize an accurate microbiological diagnosis, clinicians should ensure that diagnostic specimens are properly obtained and promptly submitted to the microbiology laboratory.
Initiation of antibiotic therapy pending culture and sensitivity reports
The best approach would be to rule out a life-threatening infection and if the child is stable, to wait and watch for the evolution of the illness. However, in cases, where it is reasonably certain that there is a possibility of a bacterial infection and waiting is dangerous/detrimental, initial therapy for infection is often empiric as guided by the clinical presentation, supportive lab evidences and will be modified or stopped as per the reports. Initiation of antibiotic too early, in such situation, will mask the gravity of illness, give false sense of security and results in poor outcome.
Interpretation of culture and sensitivity patterns to guide antibiotic therapy further
-
When an organism is identified, it is very crucial to start antibiotics pointed toward true pathogen. Contaminants and commensals should never be treated
Contaminants are the organisms from skin, unsterile instrument whereas colonizers are the organisms from non- sterile body parts, catheter, endotracheal tube, etc., and do not cause infection. Some of the examples are as under
S. pneumonia and meningococci from nasopharynx
Gonococci from vaginal secretions
E. coli from stool or rectal swab
Pseudomonas in the endotracheal tube
Candida in urine
Staph from throat
CONS.
When a pathogenic microorganism is identified in clinical cultures, the next step performed is antimicrobial susceptibility testing (AST). AST measures the ability of a specific organism to grow in the presence of a particular drug in vitro and it indicates that the isolate is likely to be inhibited by the usually achievable concentration of a particular antimicrobial agent when the test is performed using guidelines established by the Clinical and Laboratory Standards Institute.[9] Data are reported in the form of minimum inhibitory concentration (MIC), which are the lowest concentration of an antibiotic that inhibits visible growth of a microorganism after an overnight incubation, and are interpreted by the laboratory as “susceptible,” “resistant,” or “intermediate,” according to Clinical and Laboratory Standards Institute criteria. Some laboratory interprets susceptibility in terms of “zone of inhibition” and is presented as zone diameter. However, such methods are erroneous and hence MIC based reports are always recommended in clinical practice. However, one should not blindly follow the sensitivity reports and should be guided by the clinical condition of the child.
As described below, AST has some limitations that should be kept in mind.[10]
In vivo and in vitro mismatch – Some organisms carry enzymes that, when expressed in vivo, can inactivate antimicrobial agents to which the organism shows in vitro susceptibility. For example, ESBLs in Enterobacteriaceae are enzymes that mediate resistance to almost all β-lactam agents except carbapenems (e.g., meropenem or imipenem). The production of ESBL should also be suspected when treatment with β-lactams fails despite apparent in vitro susceptibility
One should also remember that in vitro sensitivities do not always result in clinical cure (e.g., aminoglycosides cannot cure enteric fever even though the report always shows salmonella sensitivity to all of them)
Clinical laboratories may provide different AST interpretations for different sites of infection (e.g., meningitis and non-meningitis AST results for S. pneumonia).
Empiric therapy
In certain cases, the empirical use of antibiotics may be scientifically acceptable, if few prerequisites are judiciously met with. These include clinically near-certain bacterial infections with or without indirect evidence of infection as determined by leukocytosis (or leukopenia in neonates), acute phase reactants (such as CRP, and procalcitonin), detection of antigen, serological tests, radiology (consolidation on X-ray), and exudates (pleural fluid, CSF, joint aspirates, abscesses, etc.). In such cases, antibiotics can be used without awaiting definite identification of the causative organism after sending investigations aimed at making a microbiological diagnosis (if available and feasible). Ideally, all empiric and preemptive therapy should end up to definitive therapy.
The followings are situations where empiric therapy is justified.
Clinically certain bacterial infection
In certain clear-cut clinical situations, specific microbiological tests are not typically performed, and one is justified to start antibiotics at a first stretch. Here, single antimicrobial agents with a narrowest spectrum should be directed at the most likely pathogens for the duration of therapy for clinically certain bacterial infections such as community-acquired pneumonia, otitis media, diphtheria, bacillary dysentery, acute lymphadenitis, or cellulitis in the ambulatory setting.
High probability of bacterial infection while waiting for lab results
In stable clinical circumstances, antimicrobial therapy should be deliberately withheld until appropriate specimens have been collected and submitted to the microbiology laboratory. Important examples of this principle are suspected of enteric fever, urinary tract infection, infective endocarditis, osteoarticular infections, etc. Patients with these infections are frequently ill for a period of several days to weeks, and in such situations, antibiotic therapy should be delayed until multiple sets of blood cultures have been obtained.
High probability of bacterial infection and waiting is dangerous
In critically ill patients, such as those in septic shock, febrile neutropenic patients, and patients with suspected bacterial meningitis, sick looking neonate empiric therapy, often with a broad coverage, should be initiated as soon as possible, once appropriate diagnostic specimens have been collected. In these situations timing of first dose is very vital and it often decides the overall outcome. It has been shown that delayed and/or inadequate therapy for infections in critically ill, hospitalized patients are associated with poor outcomes, including greater morbidity and mortality, as well as increased length of stay.[11] Therefore, a common approach is to use broad-spectrum antimicrobial agents as initial empiric therapy (sometimes with a combination of antimicrobial agents) to cover multiple possible pathogens commonly associated with a given clinical situation. This is true for both community- and hospital-acquired infections. Once microbiology results have helped us to detect the etiologic agent with or without antimicrobial susceptibility reports, every attempt should be made to narrow the antibiotic spectrum (de-escalation). This is very crucial component of antibiotic stewardship because it can reduce the cost and toxicity and prevent the emergence of AMR in the community.
Atypical course of a viral infection
The atypical course of a viral disease may indicate the likelihood of post-viral bacterial complications, and it is justified to start antibiotic empirically after drawing appropriate samples, for example, post-measles pneumonia, H1N1, and COVID 19.
Choosing an empiric antibiotic
1. Establishing a clinical diagnosis: Viral or bacterial?
A clinical diagnosis most often helps us to predict causative pathogens fitting into a clinical syndrome which would tailor the correct antibiotic rather than blindly relying on fever, WBC counts, CRP, procalcitonin, cultures, or radiology to make a diagnosis of infection.
Fever is a cardinal symptom of infection. This infection can be viral, bacterial, or otherwise. Viral infections are disseminated through body systems (e.g., upper as well as lower respiratory tract), may affect multiple systems (respiratory and gastrointestinal), and generally spread from and to close contacts.
In short duration illnesses, the fever pattern gives us the diagnosis in the majority of the patients [Table 1].
| Viral | Bacterial |
|---|---|
| Affects multiple mucosal systems of body | Bacterial infection is localized to one system or organ |
| Fever high at onset but settles within next 5 days | Fever moderate at the start, peaks by 4–5 days |
| Child comfortable during inter- febrile period | Child looks sick and often toxicduring interfebrile period |
| Similar cases in family and community | Draining lymph node often enlarged |
| CBC not contributory, as polymorphonuclear preponderance is seen in first 2 days of viral illness | |
| Antibiotics – no role | Narrow spectrum appropriate single antibiotic |
Viral infection
High grade at the onset, fair response to paracetamol, a normal inter-febrile period and a rhythmic fever (comes up as soon as the antipyretic action of the drug wanes), the appearance of cold, cough on days 2–3, and decreasing fever by days 3–4. However, atypical progress in a suspected viral infection may a complicating bacterial infection, and antibiotics may be justified in such cases, for example, post- measles pneumonia, and pneumonia complicating flu.
Bacterial infection
Fever moderate to high at onset, poor response to paracetamol, sick inter-febrile period, and fever not abating or worsening by days 3–4. This suggests a localized bacterial infection. We should look for the focus of infection such as tonsillitis, urinary tract infection, adenoiditis, or bacillary dysentery. Once focus is identified that empirical antibiotic is justified.
Mild-to-moderate fever at the onset, child getting sicker in the inter-febrile period as the days progress, poor response to antipyretics, and trend of fever worsening by days 3–4. This suggests a bacteremic bacterial infection. It may localize to the lungs (pneumonia) or meninges (meningitis) by days 3–4 or may not localize (typhoid fever). In such situation, an empiric antibiotic is started after appropriate samples for culture, and other investigations are obtained.
At the same time, it is very important to remember non- infectious causes of fever such as Kawasaki disease, rhreumatological disorders, malignancy, dehydration fever, heat fever, and drug fever. Fever may also be absent in the presence of bacterial infection in certain situations such as sick neonate, immune-compromised child, whooping cough, chronic sinusitis, and tuberculosis.
Once it is reasonably certain that one is really dealing with a possible bacterial infection, an attempt should be made to select the most appropriate antibiotic, while making such decision following factors are considered, [Table 2].
| Host related factors | Organism and drug related factors |
|---|---|
| Clinical presentation including site of infection, and severity of the disease. | Microbial local drug sensitivity pattern |
| Age | Microbial drug resistance pattern |
| Nutrition | Local epidemiology |
| Immune status comorbidity | Pharmacokinetic and pharmacodynamic aspects of drug |
| Vaccination | |
| Prior antibiotic usage/admission/procedure/device |
2. Which is the likely organism? Which antibiotic is appropriate for the given situation?
We need to know the likely pathogens generally causing a particular illness in a particular age group. Age and site of infection are very crucial to predict the offending bug, for example, pneumonia in a school going child; acute otitis media in a toddler; and sepsis in a neonate. Having considered the possible pathogenic organisms, think about the antibiotics to which each of these organisms is sensitive.
One may not undergo this exercise every time. It will be very easy and practical to follow protocols and guidelines suggested by various organizations such as WHO, CDC, IDSA, and IAP [Tables 3 and 4]. Remember that it should be evidence based and as per your local epidemiology and drug susceptibility pattern.
| Tonsillopharyngitis | GABHS, Corynebacterium diphtheriae |
| Acute otitis media/sinusitis | S. pneumonia, H. Influenzae B,Moraxella catarrhalis |
| Pneumonia | S. pneumonia, H. Influenzae B, Staphylococcus aureus, Mycoplasma |
| Enteric fever | Salmonella Typhi, Paratyphi |
| Dysentery | Shigella |
| UTI | E. coli, Proteus, Klebsiella |
| Pyogenic meningitis | S. pneumonia, H. Influenzae B,Neisseria meningitides |
S. pneumonia: Streptococcus pneumonia, H. Influenzae: Haemophilus Influenzae
| Condition | Associated features | Remarks | Antibiotic choice |
|---|---|---|---|
| Tonsillopharyngitis | Fever, throat pain, exudates on tonsils, lymph node± | Age usually above 4 years | Amoxicillin for 10 days 40 mg/kg |
| AOM | Fever, excessive crying, URTI, ear discharge, otalgia | Otoscopy must | Amoxicillin 40 mg/kg for 10 days for <2 years, otherwise 7 days |
| Sinusitis | Fever, cough, nasal discharge | Often underdiagnosed | Amoxicillin 40 mg/kg for 10 days |
| Pneumonia | Rapid breathing | ||
| Cough | Admit age <3 months severe disease oral switch as soon as possible | Amoxicillin 40 mg/kg or amoxiclav or ceftriaxone or cefotaxime 100 mg/kg for 5–7 days | |
| Cellulitis, impetigo, pustulosis | Skin lesions | Topical mupirocin only in the absence of systemic manifestation and localized lesions | Cephalexin/cefadroxil 30–50 mg/kg for 5 days |
| lymphadenitis | Tender swelling | Check for recurrence, other lymph nodes, organomegaly, anemia chronicity general well-being, growth | Cephalexin/cefadroxil 30–50 mg/kg for 7 days |
| Bacillary dysentery | Macroscopic blood in stool | Toxemia temp tenesmus | Cefixime 8–10 mg/kg for 5 days |
| UTI | Urinary complaints, may may present fever without focus | Culture always, USG always, MCU as indicated, DMSA scan as indicated | Cefixime, amoxicillin for 7–10 days. Prophylaxis may be required |
| Typhoid fever | Fever, pain in abdomen, vomiting, toxemia | Admit if needed, blood culture must | Cefixime 20 mg/kg for 14 days ceftriaxone100 mg/kg/day in admitted cases |
| Pyogenic meningitis | Fever, irritability, convulsion, vomiting, altered senses | Always IV, no oral switch, duration depends upon organism | Ceftriaxone 100 mg/kg plus vancomycin* for 7 – 10 days depending on the bug. Complete for 2 weeks if no bug is isolated |
In most of the community acquired bacterial infections above the diaphragm amoxicillin or at the most amoxiclav is sufficient. For suspected staphylococcal pneumonia cloxacillin or cefazoline is recommended. For methicillin-resistant Staphylococcus aureus one can use vancomycin or linezolid. In bacterial infections below the diaphragm third generation cephalosporin is justified. Vancomycin until culture sensitivity report, in view of rising incidence of DRSP in meningitis*
Hospital-acquired infections or nosocomial infections are also a global health issue and have different epidemiology and varied dynamics. The rate of nosocomial infections is higher in developing countries than in developed world. They are associated with cause prolonged stay, disability, and financial burden. Most prevalent hospital associated infections include central line-associated bloodstream infections, catheter- associated urinary tract infections, surgical site infections, and ventilator-associated pneumonia. They are commonly caused by drug-resistant organisms, both Gram-positive (e.g., methicillin-resistant Staphylococcus aureus [MRSA]) and Gram-negative (e.g., Pseudomonas aeruginosa) bacteria, which are often endemic in hospitals [Table 5].
| Vascular related bloodstream infection | CONS, enterococci, MRSA, Enterobacter, Pseudomonas |
|---|---|
| Shunt infection | CONS, MRSA, Propionibacterium acnes |
| Urinary catheter-related infections | E. coliand Gram-negative bacilli |
| VAP/HAP early onset | Enterobacteriaceae, Haemophilus, MSSA, S. pneumonaie |
| HAP late onset | Pseudomonas, Acinetobacter,MRSA |
MRSA: Methicillin-resistant Staphylococcus aureus
In selecting empiric antimicrobial therapy for such infections, one should consider the following factors: (1) The site of infection; (2) the organisms most likely to be colonizing that site. It is well-known that, intravascular catheter-related bacteremia is frequently a result of colonization and infection caused by staphylococci present on the skin; (3) colonization with a known organism (e.g., a screening nasal swab for MRSA) before admitting patients to the intensive care unit in situation where MRSA is highly prevalent or an outbreak situation; and (4) the local bacterial resistance patterns or antibiograms when available.
Ensure chosen antibiotic has adequate tissue penetration at the site of infection. Antibiotic penetration at the site of infection is very crucial especially in severe life-threatening infections. An antibiotic that penetrates well into the meninges is a pre-requisite for treating meningitis or sepsis in a neonate. BL-BLI and to some extent Vancomycin has poor penetration in the brain and lungs, respectively, therefore may not be a good choice for meningitis or pneumonia.[12] Excretion of the antibiotic through the biliary tract is an advantage for treating cholangitis or enteric fever where the bacteria sequestrate in that tract. Furthermore, aminoglycosides have poor penetration into the cells and therefore are unsuitable for treating infections where the organisms are primarily intracellular, for example, Salmonella. Antimicrobial concentrations attained at some sites (e.g., ocular fluid, CSF, abscess cavity, and bone) are often much lower than serum levels. For example, first- and second-generation cephalosporins and macrolides do not cross the blood–brain barrier and are not recommended for the central nervous system infections. Daptomycin, an excellent bactericidal agent against gram-positive bacteria, is not useful for the treatment of pneumonia (e.g., pneumococcal pneumonia) because it is inactivated by lung surfactant.[13] Sometimes antibiotics belonging to the same class have different clinical implications, for example, quinolone group of antibiotics. Moxifloxacin does not achieve significant urinary concentrations, making it unsuitable for the treatment of UTIs. Levofloxacin and ciprofloxacin are excellent choices for UTIs caused by susceptible bacteria. Some quinolones such as levofloxacin and moxifloxacin are also used as respiratory quinolones while others are not. In addition, route, dose, and duration of therapy will also be decided by site of infection, for example, meningitis, septic shock, and febrile neutropenia where parenteral antibiotics for relatively longer duration is needed.
1. What is the route, frequency, and duration?
Route
Intravenous administration is compulsory in seriously ill patients where predictable concentration of drug is required such as bacterial meningitis, infective endocarditis, and neonatal sepsis. Patients with active infections on parenteral antibiotics, once show signs and symptoms of improving clinical status or resolving, can be switched on oral therapy. In invasive infections such as pneumonia, pyelonephritis, or abscesses, oral formulation should be appropriately selected which has an excellent absorption and bioavailability and minimal GI side effects. Gastrointestinal bleeds, intolerance to oral medications, and adherence issues are the contraindications for oral route.
Please remember that more serious infections, in which high serum or CSF drug concentrations are desired, a switch to oral therapy is less reliable and not generally recommended. Such conditions include neonatal sepsis, bacterial meningitis, infective endocarditis, febrile neutropenia, brain abscess, and orbital cellulitis.
Dosing interval
This is very crucial factor as it helps
To optimize empiric therapy
To maintain therapeutic levels
To reduce toxicity
To prevent AMR.
Certain antibiotics such as β-lactams and vancomycin exhibit time-dependent activity [Figure 2]. They have relatively slow bactericidal action and are effective because of the extensive amount of time the antibiotic binds to the microorganism. Hence, for this group of antibiotics their serum concentration must exceed the MIC for the duration of the dosing interval, and this can be optimized either through continuous infusion or frequent dosing. On the other hand, certain antibiotics such as aminoglycosides, fluoroquinolones, metronidazole, and daptomycin exhibit concentration-dependent killing [Figure 3]. They achieve high concentrations at the binding site which eradicates the microorganism and their bactericidal activity is enhanced as the serum concentration is increased. With these agents, the “peak” serum concentration, are more closely associated with efficacy. The frequency of the dosing interval does not matter significantly.
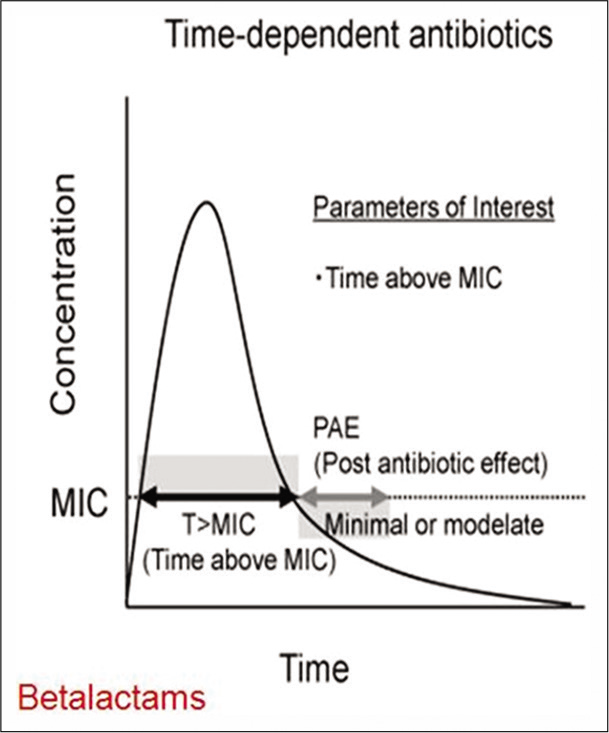
- Time dependent activity of antibiotics.
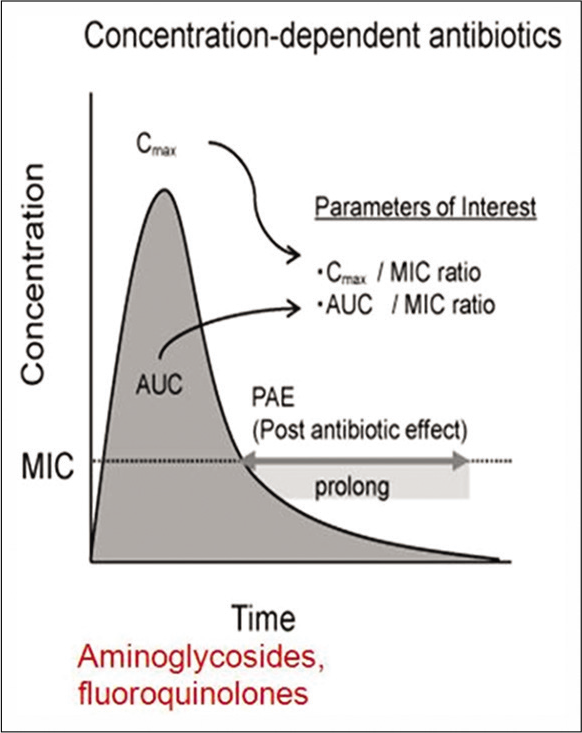
- Concentration dependent activity of antibiotics.
Duration of antibiotic therapy
It is very important to optimize the duration of antibiotic therapy. The duration should be optimum to achieve desired therapeutic response with no or minimal side effects and to prevent resistance as well. Longer than necessary courses carry higher risks of adverse effects, non-adherence issues, selection pressure, and high cost. A number of studies have stressed an emphasis on shorter courses of therapy, especially in community acquired infections [Table 6].[13]
| Disease | Treatment, days | |
|---|---|---|
| Short | Long | |
| Community-acquired pneumonia | 3–5 | 7–10 |
| Nosocomial pneumonia | ≤8 | 10–15 |
| Pyelonephritis | 5-7 | 10–14 |
| Intra-abdominal infection | 4 | 10 |
| Acute bacterial sinusitis | 5 | 10 |
| Cellulitis | 5–6 | 10 |
Use of rational antimicrobial combinations
Most of the time in clinical practice single-agent antimicrobial therapy is generally preferred. Antibiotic combinations of two or more agents are recommended in few selected cases.
For synergistic activity against a microorganism
Synergistic action implies that in vitro the combined effect of the antimicrobial agents is greater than the sum of their independent activities measured separately.[14] For example, the combination of certain β-lactams and aminoglycosides exhibits synergistic activity against a variety of Gram-positive and Gram-negative bacteria and is used in the treatment of serious infections, for which rapid killing is essential (e.g., treatment of endocarditis caused by Enterococcus species with a combination of penicillin and gentamicin). Here, penicillin alone is only bacteriostatic, and gentamicin alone has no significant activity but when gentamicin is used with penicillin, the combination imparts much desires bactericidal action. Similarly for endocarditis due to S. viridans, a combination of penicillin or ceftriaxone with gentamicin results in a more rapid clearance of microorganisms. With this synergistic combination 2-week duration of therapy can be as effective as penicillin or ceftriaxone used alone for 4 weeks.
In a critically ill patients empiric therapy before microbiological etiology and/or antimicrobial susceptibility can be determined
Combination therapy is used in this setting to ensure that at least one of the administered antimicrobial agents will be active against the suspected organism(s). For example, when a patient has been hospitalized for serious septic shock, it would be appropriate to provide initial therapy with two agents that have activity against both Gram-positive and Gram-negative organisms.
For treatment of polymicrobial infections
Certain infections are caused by more than one organism. In such instances a combination regimen may be preferred for a comprehensive broader spectrum to target possible polymicrobial agents, intra-abdominal infections brain abscess, necrotizing fasciitis, etc., are poly microbial infections. Antimicrobial combinations, such as a third- generation cephalosporin or a fluoroquinolone plus metronidazole, can be used as a potential treatment option in intra-abdominal infections and can be more cost-effective than use of single molecule like carabapenem.
To prevent emergence of resistance
The emergence of AMR is generally the result of selective pressure from antimicrobial therapy probability of emergence of resistance against two drugs is lower as compared with a single drug. While using combination therapy we are reassured that at least one drug will be effective, and this in turn prevents the resistant mutant population from emerging as the dominant strain. The classic examples for such combination drug therapy include use of more than 3 or 4 drugs in the treatment of infections requiring long duration therapy such as tuberculosis and the human immunodeficiency virus (HIV). Prolonged treatment duration is likely to provide more chances of emergence of resistance especially when the therapeutic agents are limited.[10]
HOST FACTORS
Age
Most pediatric drug dosing is guided by weight. Children below 3 months are more prone to have serious occult bacteremia and hence intravenous antibiotic/s are warranted. Tetracyclines should be used only in older children above 8 years of age, chloramphenicol not before 2 months, and quinolones should be avoided in children under the age of 12 years. Extremes of the age are considered very crucial while selecting antibiotic/s class and their dosages. These groups of patients handle drugs differently, due to differences in body size and kidney function and they often have very narrow safety window.
Renal and hepatic function
Efficiently working kidney and the liver are often prerequisite during antimicrobial administration. These are the primary organs responsible for the elimination of drugs from the body. Dose adjustments are advocated to prevent accumulation and toxicity in patients with reduced renal or hepatic function. For example, aminoglycoside in during compromised renal function ceftriaxone in jaundiced neonates.
Genetic variation
Genetic susceptibility to the adverse effects of antimicrobial agents, which has been demonstrated for several antimicrobial agents, is occasionally significant enough to warrant testing for such variability before administration of certain drugs, classic example is that of glucose-6-phosphate dehydrogenase deficiency, which can result in hemolysis in individuals when exposed to certain antimicrobial agents, such as dapsone, primaquine, and nitrofurantoin.
History of allergy or intolerance
A history of antimicrobial allergy or intolerance should be routinely obtained in the evaluation and management of infection. A history of drug allergy may preclude the use of certain groups of antibiotics such as penicillins or sulfa.
History of recent antimicrobial use
Eliciting a history of exposure to antimicrobial agents in the recent past (approximately 3 months) can also help in selection of antimicrobial therapy.[15] The causative microorganism for a current episode of infection is likely to be resistant to the drug and/or drug class which has been used recently due to selection pressure. Here, it would be prudent and logical to use an alternative agent. For example, the emergence of MRSA and ESBL in a child has previously prescribed third-generation cephalosporin or quinolones.
Assessment of response to treatment
Desired successful therapeutic response is associated with improvement in vital signs, clinical parameters, and investigation findings. These include improving symptoms and signs (e.g., a decrease in fever, tachycardia, or toxemia), laboratory values (e.g., decreasing leukocyte count, CRP etc.), and radiologic findings (e.g., decrease in the size of an opacity). However, radiologic improvement can frequently lag behind clinical improvement, and routine radiographic follow-up of all infections is not always necessary.
If one judges that the empirical antibiotic has failed, it is important to reassess the diagnosis and also to rule out non- infective causes. Even in reasonably diagnosed bacterial infections, if one change of antibiotic fails, it is best to search for an alternative diagnosis or complications.[8,15]
ANTIBIOTIC DE-ESCALATION
It is a key element within antimicrobial stewardship programs. When microbiological information is not available yet, the use of broad-spectrum antimicrobial(s) constitutes the backbone of the empirical therapy in critically ill patients. De-escalation refers to the reassessment of treatment when culture results are available, and it generally incorporates a reduction in the spectrum of administered antibiotics either by discontinuation of antibiotics or switching to an agent with a narrower spectrum. Hence, once the pathogen(s) are identified, the empiric antibiotic(s) should be stopped or reduced in number and/or narrowed in spectrum. This strategy appears to be, capable of promoting therapeutic appropriateness, averting AMR and reducing toxicity and costs.
Failure of an antibiotic
In an immuno-competent host with community acquired infection most of the time correctly selected antibiotic works. If it does not work assess the dose, duration, compliance, and adherence. Once all these factors are reassessed, it is irrational to continue trial with different empirical antibiotics and it is not ideal to add or change antibiotics empirically. Do not go on adding the antibiotics and expand the spectrum. Try to find out the cause of a failure of an antibiotic prescription.
The common causes for such a failure are as below.
Collection of pus: Empyema, subdural collection
Foreign body: Nonresolving/recurrent pneumonia
Necrotic tissue: Polytrauma, burns, SSSS, TEN, and SJ syndrome
Different organism: Mycoplasma, TB, viral, leptospirosis, rickettsial disease, and fungal
Improper diagnosis: Non-infectious cause like Kawasaki disease
Antibiotic-resistant bacteria: MDR TB, MRSA, and ESBL
Immunocompromised HOST: HIV,
Special situations: Cystic fibrosis, asplenia, and splenectomy.
Please note that the maintenance of vitals, including oxygenation, perfusion, euglycemia, asepsis measures, etc., is prerequisite for favorable outcome. Any abscess needs to be drained, offending material such as foreign body, and catheter needs to be removed, and in the skin and soft-tissue infections, debridement of necrotic tissues with meticulous wound care are important aspects of successful antibiotic therapy on individual case based situation. It is important to realize that recurrent bacterial infections are always a result of some background abnormality which may be anatomical, functional (mucociliary), or immunological. Hence, it should be a rule to thoroughly investigate a case where repeated bacterial infections occur or when it fails to respond.
CONCLUSION
Irresponsible and overuse of antimicrobials are one of the world’s most vital public health problems. Microbes adapt to the antimicrobials easily in no time resulting into emergence of AMR. People infected with such antimicrobial-resistant organisms are more prone to have serious disease with longer and expensive hospital stays, are often victims of infection associated complications and more likely to die as a result of a serious infection. It is very important to be an antimicrobial steward by practicing responsible antibiotic therapy. Antibiotic stewardship promotes the appropriate use of antimicrobials (including antibiotics) to improve patient outcomes. It is also an attempt to reduce the emergence and spread of infections caused by multidrug-resistant organisms.
Important considerations when prescribing antimicrobial therapy include obtaining an accurate diagnosis of infection and understanding the difference between empiric and definitive therapy. One should identify every opportunity to stop or to switch to narrow-spectrum, cost-effective oral agents for the shortest duration keeping type and site of infection, pharmacological characteristics of drug and host factors in mind. This will result into favorable outcome with no or minimal adverse effect and reduced chance of emergence of AMR.
Take home message
Try to obtain an accurate microbial diagnosis
Prescribe an antibiotic when it is absolutely needed, i.e., in the presence of true bacterial infection
Always prescribe first line, possible narrowest spectrum of antibiotic for optimum duration in optimum dosages
Assess the response and look for side effects
Avoid overuse of antibiotics/irrational combinations
In case of non-response look for compliance, complications, and alternative diagnosis
Do not use antibiotic/s in nonbacterial conditions
Develop culture of culture
In health-care associated infections start broad spectrum and de-escalate/revise the regimen as per culture and sensitivity reports
Observe infection control measures – hand hygiene is the cheapest and most cost effective
Improve vaccination coverage
Try to establish microbial diagnosis by other point of care non culture methods
Self-audit your antibiotic usage.
Let us understand the importance of antimicrobial stewardship and observe self-discipline in antibiotic prescription to combat the ever expanding menace of AMR.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Prevention and Containment of Antimicrobial Resistance. Available from: http://www.ino.searo.who.int/linkfiles/other_content_whd11-seminar_presentation-wrpdf [Last accessed on 2020 May 20]
- [Google Scholar]
- Challenges of antibacterial discovery. Clin Microbiol Rev. 2011;24:71-109.
- [CrossRef] [PubMed] [Google Scholar]
- Extending the Cure. 2007. Policy Responses to the Growing Threat of Antibiotic Resistance. Washington, DC: Earthscan; Available from: http://www.rff.org/publications/pages/publicationdetails.aspx?publicationid=9575 [Last accessed on 2020 May 20]
- [Google Scholar]
- Treatment Guidelines for Antimicrobial Use in Common Syndromes. 2019. Available from: https://www.icmr.nic.in/sites/default/files/guidelines/treatment_guidelines_2019_final.pdf [Last accessed on 2020 May 20]
- [Google Scholar]
- High level aminoglycoside resistance and reduced susceptibility to vancomycin in nosocomial enterococci. J Glob Infect Dis. 2010;2:231-5.
- [CrossRef] [PubMed] [Google Scholar]
- Rationalizing antibiotic use to limit antibiotic resistance in India. Indian J Med Res. 2011;134:281-94.
- [Google Scholar]
- Judicious antimicrobial therapy in pediatrics, when and what? Pediatr Infect Dis. 2009;1:14-9.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. 2020. Available from: http://www.clsi.org/am/template.cfm?section=about_clsi [Last accessed on 2010 Dec 16]
- [Google Scholar]
- General principles of antimicrobial therapy. Mayo Clin Proc. 2011;86:156-67.
- [CrossRef] [PubMed] [Google Scholar]
- Inadequate antimicrobial treatment of infections: A risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462-74.
- [CrossRef] [PubMed] [Google Scholar]
- Bacterial meningitis in children. Pediatr Clin North Am. 2005;52:795-810.
- [CrossRef] [PubMed] [Google Scholar]
- The new antibiotic mantra-shorter is better. JAMA Intern Med. 2016;176:1254-5.
- [CrossRef] [PubMed] [Google Scholar]
- Section E: Anti-infective therapy: Principles of anti-infective therapy. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 2010;1
- [Google Scholar]
- Inhibition of daptomycin by pulmonary surfactant: In vitro modeling and clinical impact. J Infect Dis. 2005;191:2149-52.
- [CrossRef] [PubMed] [Google Scholar]






