Translate this page into:
MedTech Innovation using a structured Biodesign process: Barriers and Opportunities
*Corresponding author: Gunda Srinivas, Consultant, Pediatric Emergency and Pediatrics, Aster RV Hospital, JP Nagar, Bengaluru, Karnataka, India. srinivaspapadoc@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chaturvedi J, Srinivas G. MedTech Innovation using a structured Biodesign process: Barriers and Opportunities. Karnataka Paediatr J 2021;36(2):106-12.
Abstract
Objectives:
Medical Technology (MedTech) can be defined as the application of science to develop solutions to health problems. It also includes devices, processes and existing systems in the healthcare ecosystem. Biodesign process is the tried and tested methodology of identifying the unmet clinical needs and solving the problems of the healthcare ecosystem by applying science and technology. Hence, biodesign process is nothing but a process of developing systematic MedTech Innovations. Just like there is clinical research for disease and all its aspects like etiology, management etc, the systematic process of identifying problems and finding solutions in healthcare ecosystem is termed biodesign process. The starting point in this whole process is to define the right problem, figuring out all the possible solutions, zeroing on to the right solution and see that it solves the problem efficiently. But ultimately, did that solve the problem? At the first instance, was there a problem at all? These are the questions that arise during the course and biodesign process has the answers to all these questions. This process allows the innovator to ask the right questions and find the right answers in the best possible way, so that any of the time and effort of the team are not futile. The biodesign process established by the Stanford biodesign program gave the basic understanding of the process, which was modified to the Indian healthcare ecosystem to identify relevant problems and innovate suitable solutions.
Materials and Methods:
Multidisciplinary teams went through clinical immersions, figured out various crucial needs, validated their understanding with subject experts, brainstormed about the new strategies/solutions/ approaches, and then did the prototyping of these solutions.
Results:
71 detailed observations made overall of which 52 critical and unmet clinical problem statements with significant negative impact on patient outcome were obtained.
Conclusion:
A structured biodesign process with active role of the clinician at every stage gives better insights into the unmet clinical needs in the healthcare ecosystem.
Keywords
Biodesign process
Health innovation
Stanford biodesign process
AIM program
Pediatric innovation
INTRODUCTION
Approximately 75% of all the medical equipment and diagnostic devices that are being used in India are imported from many of the developed countries, which makes healthcare costly. This has proven to be a bottleneck as witnessed in the current COVID crisis as well when there was a sudden increase in the need of many such devices to be deployed across the healthcare delivery ecosystem at a rapid pace. Furthermore, many a times, they are not adapted to the needs of India’s unique healthcare ecosystem which include the healthcare provider’s approach to care, variable sourcing needs, and who exactly reimburses the cost of healthcare delivery. Hence, currently, there is a dire need to indigenously develop medical technologies to fulfill the specific needs of our unique Indian healthcare ecosystem. Few current relevant examples are the oxygen generating plants, oxygen concentrators, ventilators, etc.
Overview of the biodesign process
Biodesign is the systematic process of innovation in healthcare systems/medical technologies. Innovation in healthcare systems is usually thought to be discovery driven where scientific research at various laboratories, universities, pharma, and biotech leads breakthroughs leading to further developments. This is the typical top-down model or the laboratory to bedside pattern. Over the last decade, there has been a major shift in this approach to a more practical bottom-up model. A deeper focus on innovation based on our unique needs has emerged as a sustainable model for medical product development, specific to India, particularly in the domain of biomedical technology (medical devices and diagnostics). Influenced by the advent of design thinking (design thinking has been dealt with in article titled “Healthcare Innovation and Design thinking” of this issue) across industries and new age organizations alike, innovators have started to focus more on having a deeper understanding of the ground level practical clinical needs as the critical starting point of the process to innovate in this domain.[2] The mantra for this biodesign process is that “a well-characterized/structured need is the DNA of a great invention.”[3]
The Stanford biodesign process
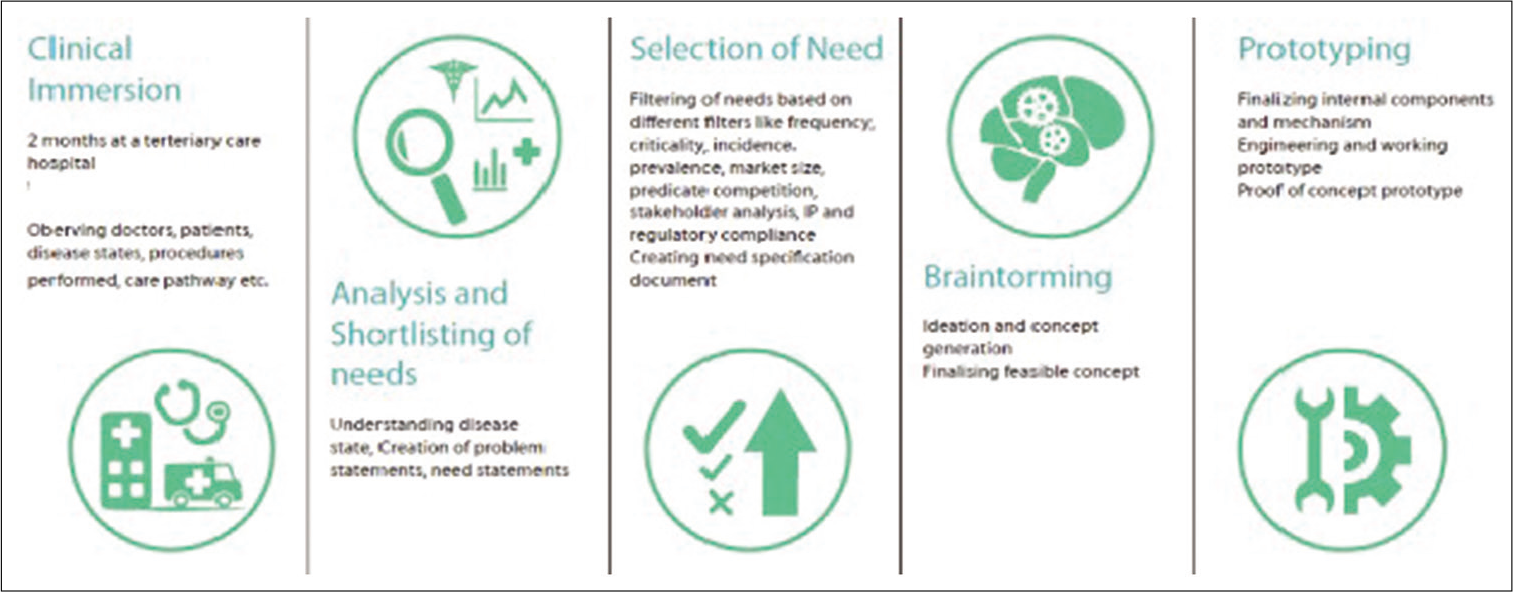
The implementation of the biodesign process started from the Stanford Biodesign program[2,4] of the well-known Stanford University through Stanford Byers Center for biodesign which began in 2001 with a primary focus to train leaders in Biomedical Innovation. The differentiating feature of this process is the priority on needs identification through clinical observations, a formal selection process, researching and characterizing needs through structured filtering process before beginning the process of inventing. The teams involved typically spend 3–5 months performing need identification and characterization before they even start thinking of a solution. The Stanford Biodesign process has 3 phases with 6 stages with various activities happening in each stage [Figure 1].

- An infographic of the three phases of biodesign process is shown here as elaborated by Stanford biodesign.
Phase of Identification
This is the phase where the unmet needs are first identified that are later clinically validated. A multidisciplinary team composed of an engineer, a product designer, a business graduate, and interestingly, a doctor who is a critical part of such teams, carryout clinical observations inside a hospital at bedside, in a specific focus area or a problematic domain of hospital such as OPD, procedure room, and laboratory for a period of 6–8 weeks. The team shadows/follows doctors, paramedical staff, and patients during this period, and keenly observes the activities between health-care personnel and patients including interactions, procedures, and the overall care provided. This is termed as clinical immersion. The team is informed to note down all the observations including seemingly efficient processes and also inefficient practices and poor health outcomes without any bias. After systematically documenting the observations for around 6–8 weeks, the team brainstorms to categorize and sort the observations using a defined process to filter out the not so relevant ones.
Once a critical need is identified, a structured document containing a set of all the needs criteria is compiled. This so-called “needs criteria” essentially is a list of must-haves and nice-to-haves in the proposed solution, which actually are the objective parameters that are expected to be satisfied from the prospective solution.
Phase of Invention
During this phase of invention, the team brainstorm all possible ways in which the need/problem can be addressed/ solved. This brainstorming session focusses on generating a large set of ideas that build cumulatively on each other. The problem statements to work on are selected based on satisfying these needs criteria initially outlined in the need specification document. In the next phase, the teams focus on the constant prototyping and validation with stakeholders.
Phase of Implementation
This stage includes evaluation of the intellectual property issues, regulatory compliances, validating the stakeholders, and strategy to adopt in marketing and also includes the plan for manufacturing process, operations, and finance. The preclinical and clinical testing of the prototype is also taken into account.
As an example of the practical application of Stanford Biodesign program in cardiology was, solving the unmet clinical need of the “detection of potential arrhythmias in non-hospitalized patients.” With a team led by cardiologist as part of the biodesign program, the top solution that filtered out was a long term (up to 14 days), water resistant, disposable patch-based monitor to identify cardiac arrhythmias. This cardiologist eventually formed a start-up and licensed the technology from Stanford, following which their team developed a complete solution, which included a cloud-based algorithm and robust supporting services. The device is currently commercially available [Figure 2]. To date, the device has been used on nearly 500,000 patients, and several publications have documented the clinical and economic utility of the approach. This device is an example of a successful medical device initially conceived by a fellowship group in the Stanford Biodesign Program. It is currently commercially available in the United States and Europe.[5]

- A novel single-use, 14-day cardiac event monitor.
Indian specific Biodesign process
The Affordable Innovation in MedTech (AIM) Program[2] is based on my (Dr. Jagdish Chaturvedi) experience with the Stanford biodesign program. There were some lacunae felt when being applied to the Indian medical ecosystem and as my role as clinical director at InnAccel, an Indian medical technology acceleration company, an improvised version of the Stanford Biodesign program was conceived with few modifications to suit our problems and solutions needed. This was the AIM program. The important additions/ modifications were as follows:
Involvement of working clinicians as clinical team members.
Collection of larger amount of data (in terms of frequency and criticality), focused on negative outcomes.
The use of pre-calibrated filters designed to bring out needs with substantial market size which can be solved by moderately complex solutions.
I (Dr. Gunda Srinivas) would like to explain the importance of the need to find out the right clinical problem and document it appropriately as well with a simple example here.[6]
A good need should address a clear and well-defined problem
A clinical problem is an undesirable situation that leads to a negative outcome. A need is the requirement to solve the problem and avoid the negative outcome. For example: “A faster way to restore blood/fluid volume to normal levels in children (under the age of 5) who are brought to the casualty/ Emergency department in a state of severe dehydration, in order to prevent hypovolemic shock.” This is a need which all the pediatricians can connect well and this statement clearly defines what the solution should focus on what? – Faster restoration of blood volume; for whom? – Under 5 children; where? – In the casualty; and why? – To prevent hypovolemic shock. The problem being addressed is the delayed restoration of intravascular volume in children with dehydration which is common due to difficult IV access. Let’s see the same clinical problem, if not constructed well or poorly observed and not documented in a structured manner. It would be something like this “A better way to manage dehydration in children” which is a poorly defined need. It does not provide any direction or clarity toward generation of a solution. In this case, there can be many ways to make the management of dehydration better which could be identifying levels of dehydration, preventing dehydration altogether, restoring hydration quickly, or by preventing hypovolemic shock which is the actual need here. Therefore, there is a possibility for the team to focus on an approach that may not be ideal to solve or might delay the process by deviating from the actual need.
A good need should not create a bias toward any particular solution
The purpose of a well-defined need statement is to provide a direction for the generation of a solution, but not the solution in itself. Consider the same example: “A faster way to give IV fluids in children with dehydration” very specifically and narrowly means a solution focused on delivering fluids in intravenous route only. Here, the invention team will be biased on thinking about solutions which are all directed toward giving fluids in intravenous route. Whereas in the rephrased need statement – “A faster way to restore blood/fluid volume to normal levels in children under 5 who are brought to casualty/ER in a state of dehydration, to prevent hypovolemic shock.” Here, restoring of blood/fluid volume can be done through Intravenous administration, through oral administration, intradermal, subcutaneous, or intraosseous routes as well. This need statement focuses on the outcome that fluid volume must be restored but does not direct how the volume must be restored. This allows the inventing team to explore all possibilities for solving the need which can be validated by the clinician in the team later.
A study was conducted at St. Johns Medical College Hospital, Bengaluru, to test the effectiveness of using such a structured process for MedTech innovation in neonatal and pediatric domain.
MATERIALS AND METHODS
A well-designed and structured process was used to chalk out unmet clinical needs, wherein multidisciplinary teams went through clinical immersions, figured out various crucial needs, validated their understanding with subject experts, brainstormed about the new strategies/solutions/approaches, and then did the prototyping of these solutions, as shown in [Figure 3]. In the first stage, a multidisciplinary team carried out simple clinical observations at bedside in a specific focus area of hospital. The team followed doctors, paramedical staff, and patients as well and keenly observed the care provided, in an unbiased manner. The team was instructed to note all observations including the inefficient practices and poor health outcomes. Then, the members of the team understood the critical and exact needs after performing deep dive into the keen observations made their indications and implications. Following this step, based on various factors such as clinical implication of the need and its criticality, frequency of the negative outcomes, and the market potential of the proposed solution, the team filtered out the various needs collected in the previous step. Once the critical need was figured out, the team then brainstormed various possible methods in which the problem could be solved without any bias. The intention of these brainstorming sessions was to generate as many number of high-quality ideas and solutions as possible to solve the critical problem. Finally, the team prototyped the most relevant solutions and validated their work with clinicians. A start-up was planned after this stage if the critical need and the suggested solution seemed to be sustainable in all ways at this point as well so that the solution is brought to the market and scaled up.
- A well-defined and structured process in MedTech innovation.
Criteria for selection of a strategic focus area
Around 20% of the world’s pediatric mortality happens in India. Under 5 children’s mortality in highest income quantile is 1.78 lakh/year in contrast to the lowest income quantile which is almost 3 times more at 5.3 lakh/year![6] There is a huge difference in mortality rates of various states of India. Kerala’s under-5 mortality rate is 14/1000 live births whereas Madhya Pradesh has 92/1000 live births.[7-9] These startling facts pushed us to undertake the clinical need analysis in the domain of child and newborn health.
Team composition
Around six clinical immersion sessions were carried out in a tertiary care hospital setup, and during these immersions, we had two biomedical engineers spending around 6 weeks shadowing and observing the doctors and paramedical staff in the department of pediatrics. The intended goal of this unique activity of clinical immersion was to identify clinical needs and genuine problems that the clinicians face, which could be later on taken up by the health technology startups grown indigenously and solve them at large scale.
RESULTS
Outcome of 2 months of clinical immersion
Outcome was quite interesting, with 71 detailed observations made overall. From this set of observations, 52 critical and unmet clinical problem statements with significant negative impact on patient outcome were obtained. The members of the team then worked up to identify the fundamentals of the disease state involved for better understanding, current treatment and management options, challenges with the current management, potential market size, business competitors in the market, regulatory clearances and requirements needed, and business models that would sustain and reimbursement methods. All the teams then worked on the need/problem statements that were documented during the initial phase of clinical immersion. This was done by applying four rounds of appropriate filters after discussion with treating pediatricians and the administration of the hospital, including the parents as well who are also the important stakeholders in the ecosystem, as shown in [Figure 4].
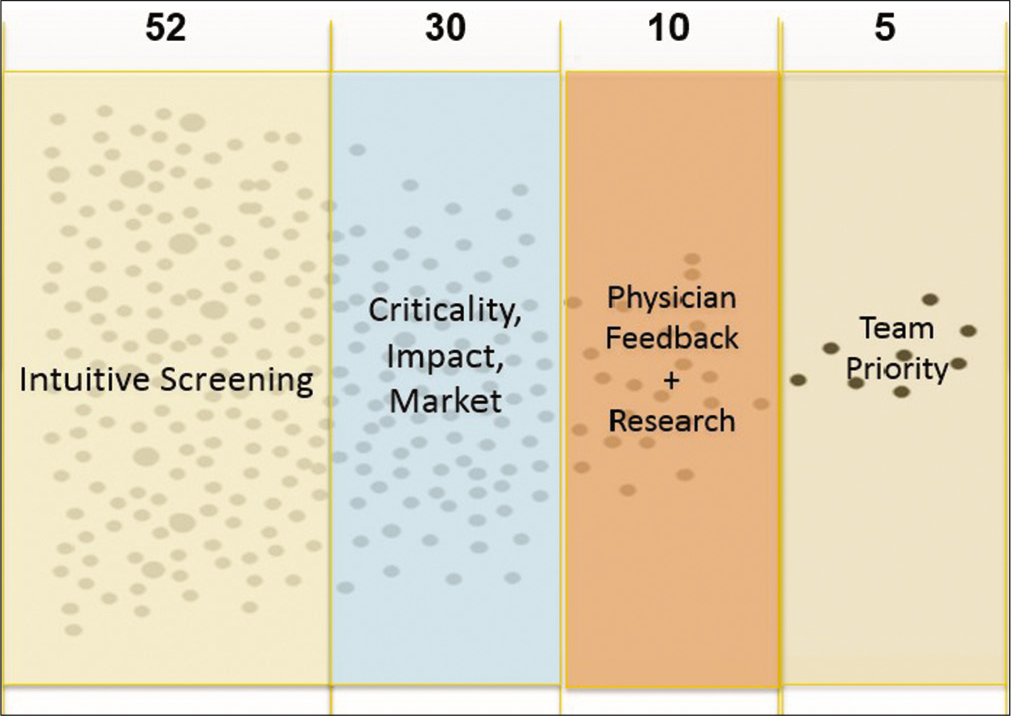
- A graphical representation of the key steps in the filtering process.
Strong motivation and personal interest are also key roles in determining the critical needs which would be pursued further, as shown in [Figure 5].
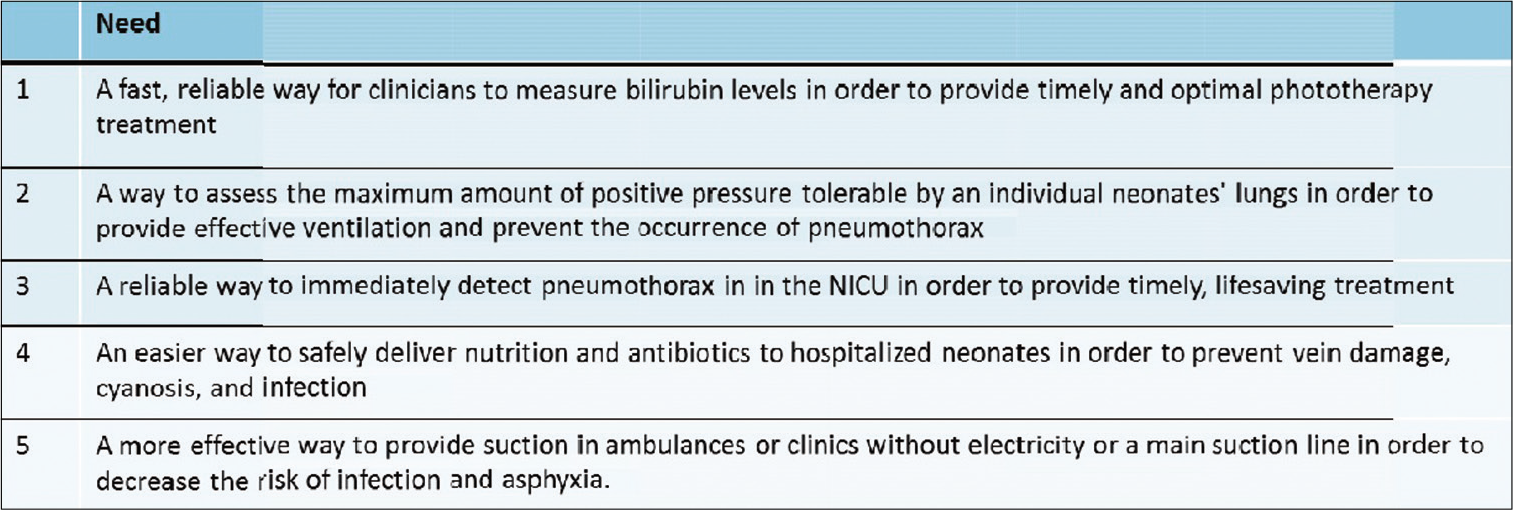
- Some of the critical needs that were identified following a structured biodesign innovation process.
DISCUSSION
This article is all about various unmet clinical needs of our unique Indian healthcare ecosystem and the ways to solve them using a well-defined and structured biodesign process. The main advantage and strength of this method were the right identification of multiple critical needs using the clinical immersion process in the shortest possible time period.
The principal barrier while using this method was the absence of a dedicated physician as part of the team which led to a number of challenges later in the process. This caused suboptimal understanding, misunderstanding, and misinterpretation of the core clinical and medical concepts such as pathophysiology of the disease and their fundamentals at a very crucial stage. A dedicated clinician as part of the team serves as a domain-specific knowledge expert during clinical immersions, helping non-clinical members to understand simple medical terminologies, relevant basic anatomy, and pathophysiological processes of the disease. He can also help during brainstorming and guide the team in selecting the appropriate possible solutions. We were forced to spend extra amount of time for extensive research and understanding by talking to various external clinicians in addition to the time spent in clinical immersion due to lack of dedicated clinician in the team.
The next challenge was an inherent sceptical attitude in the health-care ecosystem about the non-medical team members such as the engineers and salesman of the team. Many of the doctors and nurses that we met in hospitals as part of the clinical immersion became very conscious of our presence and were worried that our team was assessing their performance and monitoring their activities to report the negative aspects to the management. A dedicated clinician in the team can always build the rapport with the doctors and nurses and explain the intentions behind our keen observations and break the ice. Finally, without a dedicated clinician, it was very cumbersome to make sense of the observations and also to understand which procedures are the most critical without frequently asking the clinician in the hospital which was not preferable most of the times.
This crucial study activity depicts the importance of a pre-planned, well-structured multidisciplinary team for more effective clinical immersions with a motivated physician as an integral part of the team, bridging the gap, and guiding the team in right path. This whole activity is augmented by the presence of biomedical engineers, designers, and people unrelated to the medical systems in the team, who can look at the scenario with an unbiased and fresh perspective for better understanding and better solutions.
This might not be possible with the doctors alone, as they might get adapted and biased due to constantly working in the same setting for a long time and unfamiliar with the available technologies and systems to solve these problems. This is very crucial for the success of the end product which might be a medical device or a service that needs to fit seamlessly into the existing clinical workflow of clinicians or paramedical staff.
Spending many hours inside a hospital at bedside observing the doctors, nursing staff, and patients give a better understanding to the engineers, designers about various practical aspects of design, size, and orientation for the devices they would be designing. Ex: Nurse might have only one hand freely available while performing a specific procedure when handling children, or devices aimed toward children require specific considerations in size, positioning in ICU, bedside, etc. When the whole team is together in the clinical space, it allows more opportunities for each team member to better understand the user who might be a nurse, doctor, administrator, or the patient himself. Our experiences say that these deeper understandings and clinical insights can definitely motivate the various team members to continue to develop products and services in scenarios where others would have easily considered as not feasible. Thomas Fogarty, an internationally recognized surgeon, inventor, and entrepreneur noted, “Innovators tend to go out and ask doctors what they want rather than observe what they need. When you talk to physicians, as well as others involved in the delivery of care, you need to learn the difference between what they say and what they want, and what they would be willing to pay for, and what they actually do.”
MedTech innovation is a well-structured process that requires multidisciplinary team with collaboration across various domains. For this process to be successful, it requires complete commitment, dedication, and coordination among all team members. After initial challenges during our immersion stage, we spent couple of weeks getting to know more about the clinicians in the hospital and the department we were observing in, and attempted to recruit them to join our team for better understanding and coordination. The process went on smoothly once we had developed a good rapport with the doctors and nurses. Fortunately, we were able to course correct during mid-immersion, but we highly recommend each team to start the immersion with a multidisciplinary team, complete with dedicated clinicians, engineers, designers, etc., to effectively utilize the advantage of creative collaboration.
CONCLUSION
This structured biodesign process when employed to develop a new medical device or define a new process is highly effective in defining and innovate in the strategic clinical focus area which is essential to figure out the right problem for the appropriate solution.
Limited involvement by the clinicians in initial stages and the sceptical attitude of the doctors and nurses in hospital about the engineers were some practical challenges in the process. This proves the crucial and irreplaceable role of the clinicians not only at the very important stage of the needs assessment but also at every stage of the biodesign process.
The clinician has a new role that has emerged out of the biodesign process and all these can be managed by continuing in the clinical domain and at the same time being part of a multidisciplinary team to innovate that saves lives too.
Acknowledgments
We acknowledge our teachers, mentors, patients, and families who have given all these insights, learning, and newer opportunities to further our learning.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Health technology, quality, law, and ethics In: The New Public Health (3rd ed). Ch. 15. United States: Academic Press; 2014. p. :777.
- [CrossRef] [Google Scholar]
- A structured process for unmet clinical need analysis for medical device innovation in India: Early experiences. BMJ Innov. 2015;1:81-87.
- [CrossRef] [Google Scholar]
- Teaching biomedical innovation as a discipline. Sci Transl Med. 2011;3:92cm18.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes from a postgraduate biomedical technology innovation training program: The first 12 years of Stanford biodesign. Ann Biomed Eng. 2013;41:1803-10.
- [CrossRef] [PubMed] [Google Scholar]
- 2016. Inventing Medical Devices-A Perspective from India. :23. Available from: https://www.notionpress.com
- [Google Scholar]
- Needs-based innovation in cardiovascular medicine: The Stanford biodesign process. JACC Basic Transl Sci. 2016;1:541-7.
- [CrossRef] [PubMed] [Google Scholar]
- Case studies of innovative medical device companies from India: Barriers and enablers to development. BMC Health Serv Res. 2013;13:199.
- [CrossRef] [PubMed] [Google Scholar]
- Biodesign: The Process of Innovating Medical Technologies. New York, United States: Cambridge University Press; 2010.
- [CrossRef] [Google Scholar]
- Levels, trends and predictors of infant and child mortality among Scheduled tribes in rural India. Indian J Med Res. 2015;141:709-19.
- [Google Scholar]






