Translate this page into:
Blood counts in the children hospitalised with febrile illness in the settings of dengue endemicity – Its utility in the early recognition of the disease, Barbados
*Corresponding author: Alok Kumar, Department of Pediatrics, University of West Indies, Cavehill Campus, Queen Elizabeth Hospital, St Michael, Barbados. alokkumar.uwichill@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Krishnamurthy K, Kumar A. Blood counts in the children hospitalised with febrile illness in the settings of dengue endemicity – Its utility in the early recognition of the disease, Barbados. Karnataka Paediatr J 2023;38:8-13.
Abstract
Objectives:
In the absence of freely and timely availability of the serological test for definitive diagnosis of dengue, clinicians are often called to rely on the blood counts for making an early diagnosis of this disease. This study analyses the blood cell counts in children hospitalised in Barbados with dengue in comparison with other febrile illness (OFI).
Material and Methods:
Hospital-based and prospective study conducted over the 1-year period from January to December 2019. All the children with febrile illnesses were enrolled and tested for dengue. All these children had complete blood counts done at the time of hospitalisation, in Barbados.
Results:
There were 114 (45.4%) confirmed/probable cases of dengue and 85 (33.9%) cases of OFI among the 251 hospitalised febrile children. The total white blood cell (WBC) count had a value lower than the expected normal range in 34.6% of the cases of dengue compared to the 9.6% among the cases of OFI. The WBC counts, neutrophil counts and platelet counts were significantly lower among the cases compared to OFI. Platelet counts were lower than 150 in 28 (26.9%) dengue cases which included the 15 cases with the discharge diagnosis of dengue haemorrhagic fever. Platelet count was lower than 150 in 7 (10%) cases of OFI as well. In this study, the prevalence of thrombocytopenia was lower than those reported in the literature.
Conclusions:
Leucopenia and thrombocytopenia in febrile children at the time of their presentation insignificantly increase the risk of dengue. However, these counts on presentation may be in the normal range in a significant proportion of febrile children who confirm to be dengue.
Keywords
Paediatrics
Dengue
Blood counts
INTRODUCTION
Dengue is a global emerging infectious disease with increasing geographic distribution and magnitude in the Americas including the Caribbean region.[1,2] It is endemic throughout the Caribbean region including the English-speaking Caribbean countries.[3] Dengue affects both children and adults and is reported to be more severe in children.[4] In children, it often presents as non-specific febrile illness which sometimes progresses to the more severe form of illness such as dengue haemorrhagic fever.[5] Early recognition with close monitoring for its complications and adequate supportive management is vital to the outcome of this disease. However, clinical recognition based on the history and clinical examinations at the onset of this illness is rendered difficult due to its non-specific febrile presentation often associated with respiratory and gastrointestinal symptoms. All of which are indistinguishable from a host of other common infections seen during childhood. Confirmed or even most likely diagnosis of dengue relies heavily on the demonstration of the dengue-specific immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies in the serum of the patients, which does not reach detectable level before the 5th day of the illness or on the demonstration of the NS1 antigen in the patient’s serum, a test that is not readily and routinely available in most settings of the developing countries including the Caribbean countries.[6] In most settings where dengue is an endemic disease, clinicians are often tempted to rely on some clue from the blood counts which are routinely done in the cases of febrile illness in children, for entertaining the presumptive diagnosis of dengue in these patients at the time of presentation.
A systematic review of several retrospective studies which included both adults and children have demonstrated that patients with dengue had lower white blood cell (WBC), neutrophil and platelet counts when compared with other febrile illness (OFI).[7] However, there is a paucity of good prospective data, especially among children to back up these reports. Moreover, there are no published data on the blood counts among children with dengue from the Caribbean. Given the expected variation in the blood count response, seen in the febrile illnesses, depending on the local prevalent aetiology of the infectious illnesses among the children, it is important to evaluate the differences in blood counts among children with suspected dengue and those with OFI. Barbados, one of the English-speaking Caribbean countries with a good dengue surveillance system and a dedicated dengue laboratory provide us with an opportunity to study this difference. In this report, we have presented the findings from a prospective study on the blood count among children hospitalised with suspected and confirmed cases of dengue to demonstrate their utility in the early detection of dengue and in predicting severe forms of dengue among children.
MATERIAL AND METHODS
This is a hospital-based, prospective and observational study. This study was conducted at the Queen Elizabeth Hospital (QEH), the only hospital in Barbados which serves the whole population of this country. This hospital serves the whole of Barbados with a total population of 281,200 (2021) including 58,455 (20.8%) under 16 children. It is a public hospital and the care is free of charge to Barbadian nationals. The primary healthcare for children in this country is organised through a chain of polyclinics throughout the island which are complimented by numerous private general practitioners and paediatricians who serve as the point of entry for children in need of medical care including the febrile children. Since dengue is endemic to this country, dengue is one of the differential diagnoses in any child presenting with a febrile illness for <15 days and where there is no obvious other identifiable cause of fever. Those children with febrile illness and suspected dengue who require admission are then referred to the paediatrics department at the QEH for admission and inpatient care.
The study was designed to be a prospective clinical audit. All of the febrile (axillary temperature >37.3) children under the age of 16 years with suspected dengue who were admitted to the paediatrics ward were enrolled for this study. The period of enrolment for this study was from 1 January 2019 to 31 December 2019. At the time of admission, all the admitted children were routinely interviewed for obtaining relevant medical information including demography, duration of their illness and the constellation of symptoms followed by a detailed examination by a paediatrics house staff and a detailed investigation and treatment plan is formulated. All these children are routinely investigated with full blood counts including total white cell counts, manual differential white cell count, haematocrit, platelet counts and dengue NS1 antigen during the first 5 days of the febrile illness and dengue IgM antibody during days 6 through 15 of the febrile illness among other tests as necessitated by the clinical presentation. A discharge diagnosis is assigned at the time of discharge based on the clinical presentation and the supporting laboratory results. All these data were extracted and recorded on a separate data collection sheet specifically predesigned for this study.
IgG and IgM antibody-capture enzyme-linked immunosorbent assays (ELISAs) were performed according to the manufacturer’s instructions (Focus Diagnostics, Cypress, CA, 90630 USA). Briefly, 100 μL of 1:101 diluted serum sample or control sample is placed in a pre-washed 96-microwell polystyrene plate previously coated with anti-human antibody specific for IgM or IgG and 100 μL of antigen solution (inactivated lyophilised dengue fever virus antigen with equal amounts of DEN 1–4) was then added. Following incubation and washing, 100 μL of the substrate (tetramethylbenzidine and hydrogen peroxide) was added. Colour development was stopped with IM sulphuric acid and the optical density (OD) was read at 450 nm using a microplate auto reader. The levels of specific antibodies were calculated from OD values. Platelia™ Dengue NS1 Ag-ELISA (Biorad Laboratories, Marnes-La-Coquette, France) was used for the NS1 antigen detection. The assay uses murine monoclonal antibodies for capture and revelation. If NS1 antigen is present in the sample, an immune-complex MAb-NS1-MAb/peroxidase will be formed. The presence of an immune complex was demonstrated by a colour development and the enzymatic reaction was stopped by adding 100 μL 1N H2SO4. The OD reading was taken with a spectrophotometer at a wavelength of 450–620 nm and the amount of NS1 antigen present in an individual serum sample was determined by comparing the OD of the sample to the OD of the cutoff control serum. All blood counts were done using automated analyzers from Abbot. Differential blood counts were repeated manually.
Necessary ethical approval was obtained from the Ethics Board at the QEH and from the Institutional Review Board at the University of the West Indies (Cave Hill), Barbados. All patient information was stored in a specially designed Microsoft Access database. All data were anonymised by the authors before analysis. The outcome measured included blood count values at the time of presentation among hospitalised febrile children who were later confirmed to be dengue and those who tested negative for dengue and were considered to have OFI. Data were analysed using SPSS version 11. All calculated proportions included a 95% confidence interval (CI) which was corrected for continuity. The chi-square test (Fisher’s exact test where the numbers were small) was used for the test of significance and P < 0.05 was taken as significant. A risk ratio (RR) with 95% CI was calculated where applicable.
RESULTS
Between 1 January 2019 and 31 December 2019, there were 114 (45.4%) confirmed/probable cases of dengue among the 251 febrile children hospitalised in the paediatric unit at the QEH. There were 85 (33.9%) children where tests for dengue were negative and in 52 (20.7%) children dengue tests were not done or results could not be found. Sometimes, the test was not done due to the shortage of reagents or samples lost in transport or insufficient quantity of blood samples for analysis. In a few cases, results were not found as the laboratory request form did not have all of the identification information. Of the 114 cases of dengue, the diagnosis was based on the detection of NS1 antigen only in 27 cases (NS1 antigen testing was done in 48 cases) and based on the dengue IgM antibodies in 80 cases (dengue IgM antibody testing was done in 157 cases), whereas both the NS1 antigen and dengue IgM antibody were detected in seven cases. Most of the cases were seen during October through January, with a smaller spike in the number of cases seen during May, June and July [Figure 1]. The male-to-female ratio, the age distribution, and the mean duration of fever at the time of hospitalisation were similar among both the group of children the confirmed/probable cases of dengue and the children with OFI [Table 1].

- Seasonal distribution of the dengue cases among the children.
| Dengue | Other febrile illness | P-value | |
|---|---|---|---|
| Gender M: f | 59:55 | 44:41 | --- |
| Age | n=104 | n=85 | 0.56 |
| <1 year | 16 (14) | 12 (14) | |
| 1–5 years | 33 (29) | 37 (43) | |
| 6–10 years | 27 (24) | 17 (20) | |
| 11–15 years | 38 (33) | 19 (23) | |
| Mean duration of fever before admission | Mean-6.18 | Mean-5.46 | 0.43 |
| SD-4.70 | SD-3.54 | ||
| n=80 | n=54 |
SD: Standard deviation
The discharge diagnosis among the 114 confirmed cases of dengue among the hospitalised children was: Undifferentiated dengue fever in 53 (46.5%), classical dengue fever in 37 (32.5%) and dengue haemorrhagic fever in 15 (13.1%), and expanded dengue syndrome in 9 (7.9%).
Among the 85 cases of OFI, all were tested negative (Dengue IgM and NS1 antigen) for recent dengue infection. The discharge diagnosis was viral gastroenteritis or gastritis in 30 cases, viral respiratory infection (diagnosed based on clinical features and blood counts) including upper and lower respiratory tract in 27 cases, non-specific viral fever (based on clinical features and blood count and exclusion of other diagnosis) in 14 cases, bacterial pneumonia (diagnosed based on X-ray findings and blood counts) in six cases, bacterial sepsis (culture proven) in two cases, and other diagnosis in six cases.
The mean value of the various blood counts parameter in the dengue cases and the cases of OFI, among the hospitalised children, is shown in [Table 2]. P-values (value of <0.05 was taken as significant) from the Chi-square test of significance are also shown in [Table 2]. The total WBC count had a value below the normal range for age in 34.6% of the cases of dengue compared to the 9.6% among the cases of OFI [Table 3]. Platelet counts were lower than 150 in 28 (26.9%) dengue cases which included the 15 cases with the discharge diagnosis of dengue haemorrhagic fever. Platelet count was lower than 150 in 7 (10%) cases of OFI as well [Table 3]. When the total WBC counts at the time of presentation among the cases of dengue were correlated with the age at presentation, a significant (P = 0.001) positive correlation with a decrease in the total WBC counts was noted with increasing age [Figure 2]. Similarly, a significant (P = 0.001) positive correlation was observed with a decrease in the platelet count at presentation with the increasing age [Figure 3].
| Blood counts | Other febrile illness | Dengue cases | P-value |
|---|---|---|---|
| WBC count | |||
| Mean | 12.7 | 9.0 | 0.001 |
| SD | 8.4 | 7.6 | |
| n | 73 | 104 | |
| Absolute neutrophil counts | |||
| Mean | 7.794 | 5.114 | 0.005 |
| SD | 7.278 | 5.735 | |
| n | 57 | 89 | |
| Absolute lymphocyte counts | |||
| Mean | 3.420 | 2.782 | 0.001 |
| SD | 2.691 | 2.407 | |
| n | 59 | 87 | |
| Platelet counts | |||
| Mean | 295 | 236 | 0.5 |
| SD | 145 | 130 | |
| n | 70 | 103 | |
| Haematocrit | |||
| Mean | 33.1 | 37.7 | 0.05 |
| SD | 8.7 | 6.5 | |
| n | 54 | 88 |
WBC: White blood cells, SD: Standard deviation
| Other febrile illness | Dengue cases | |
|---|---|---|
| WBC counts | n=73 (%, 95% CI) | n=104 (%, 95% CI) |
| ≤5 | 7 (9.6, 4.7–18.5) | 36 (34.6, 26.2–44.2) |
| WBC 6–15 | 48 (65.6, 54.3–75.6) | 53 (51.0, 41.5–60.3) |
| WBC>15 | 18 (24.7, 16.2–35.6) | 15 (14.4, 8.9–22.4) |
| Haematocrit (Children < 6 years) | (n=25) | (n=46) |
| <30 | 7 (28.0, 14.2–47.6) | 0 (0.0) |
| 30–40 | 17 (68.0, 48.2–82.8) | 43 (93.5, 82.5–97.8) |
| >40 | 1 (4.0, 0.7–19.5) | 3 (6.5, 1.7–18.9) |
| Haematocrit (Children > 6 years) | (n=29) | (n=58) |
| <35 | 10 (34.5, 18.6–54.3) | 11 (19.0, 10.3–31.8) |
| 35–45 | 18 (62.1, 42.4–78.7) | 41 (70.7, 57.1–81.5) |
| >45 | 1 (3.5, 0.2–19.6) | 6 (10.3, 4.3–21.8) |
| Absolute neutrophil counts | n=57 | n=104 |
| <3000 | 16 (28.1, 17.4–41.7) | 41 (39.4, 30.1–49.5) |
| 3000–6000 | 14 (24.6, 14.5–38.0) | 37 (35.6, 26.6–45.6) |
| 6000 | 27 (47.4, 34.2–60.9) | 26 (25.0, 17.3–34.6) |
| Absolute lymphocytes counts | n=59 | n=104 |
| <1500 | 11 (18.6, 10.1–31.3) | 32 (30.8, 22.3–40.7) |
| 1500–3000 | 22 (37.3, 25.3–50.9) | 44 (42.3, 32.8–52.4) |
| 3000 | 26 (44.1, 31.4–57.5) | 28 (26.9, 18.9–36.7) |
| Platelet counts | n=70 | n=104 |
| 150 | 7 (10.0, 4.5–20.1) | 28 (26.9, 18.9–36.7) |
| 150–400 | 52 (74.3, 62.2–83.7) | 64 (61.5, 51.5–70.8) |
| 400 | 11 (15.7, 8.5–26.8) | 12 (11.5, 6.4–19.7) |
WBC: White blood cells, CI: Confidence interval
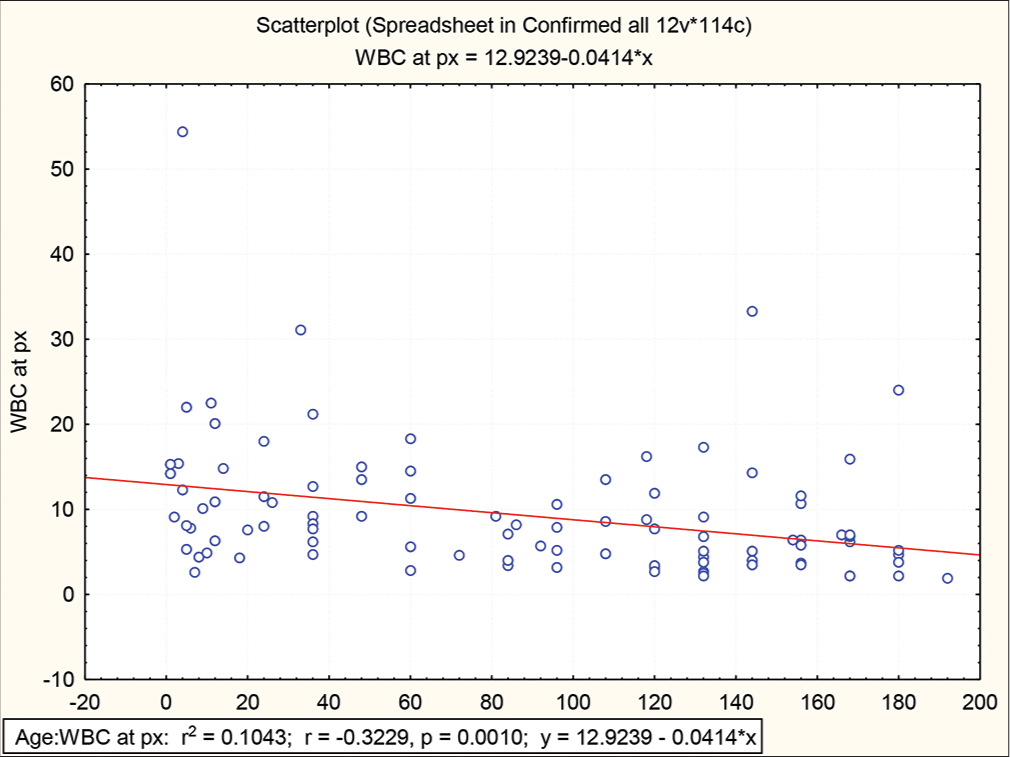
- Scatterplot of white blood cells counts against the age (in months) at hospitalisation among the cases of dengue in children.

- Scatterplot of platelet counts against the age (in months) at hospitalisation among the cases of dengue in children.
When the value of total WBC counts at presentation among those who have confirmed dengue and among those that tested negative for dengue, low total WBC counts for age and sex had a higher risk of being dengue positive (RR = 2.58; 95% CI = 1.12, 5.89; P < 0.0001). Low platelet counts (<150,000/microliter of blood) at presentation carried a higher risk of confirmed dengue (RR = 2.58; 95% CI = 1.06, 6.24; P = 0.0226). No significant difference was observed when a high haematocrit value (>40%) at presentation was used to compare those who had dengue and those that did not have dengue (RR = 0.64; 95% CI = 0.41, 1.01; P = 0.0946). The presence of both low total WBC counts for age and sex and low platelet counts (<150,000/microliter of blood) on presentation significantly increased the risk of dengue (RR = 2.91; 95% CI = 1.22, 6.95; P = 0.0067).
DISCUSSION
Dengue infection is a systemic and dynamic disease. It has a wide clinical spectrum that includes both severe and non-severe clinical manifestations.[5] Laboratory confirmation of dengue infection is crucial as the broad spectrum of clinical presentations, ranging from mild febrile illness to several severe syndromes, can make accurate diagnosis difficult. Early laboratory confirmation of the clinical diagnosis can be valuable because some patients quickly progress from mild to severe disease and sometimes death. Few studies have shown that low platelet count and decreases in WBC and neutrophils were independently associated with the presence of dengue, when compared to patients with OFI, in both adults and children.[8-10] All of these studies are from Southeast Asia and are retrospective studies with smaller sample sizes.
This is the first reported study on the blood counts in confirmed cases of dengue among children from the Caribbean region. The prospective nature and the fairly large sample size compared to the small population of this country are advantageous features of this study and permit good comparison between children with dengue and those with OFI.
Dengue is endemic to Barbados and all four serotypes have been in circulation since the early nineties.[3,11] Most cases were seen during the months of October through January which is the rainy season. This is the usual epidemiologic pattern noted in the Caribbean region.[11,12] The demographic profile and the duration of the illness at the time of hospitalisation were identical among the cases of dengue and the children with OFI who served as control.
In this study, we found that both the mean total WBC counts, mean absolute neutrophil counts and the mean absolute lymphocyte counts among the hospitalised children with dengue, at the time of hospitalisation, were significantly lower than the hospitalised children with OFI [Table 2]. Similar observations have been made in other studies of dengue in children.[7,9] However, the total WBC count may be in the normal range at the time of presentation in children hospitalised with dengue. When the range of the WBC counts was analysed among the two groups, a significantly higher proportion of children hospitalised with dengue had WBC counts <5000 (which is the lower limit of the normal range) and absolute Neutrophil counts of <3000 (the lower limit of the normal range) when compared with the children hospitalised with OFI [Table 3]. Children hospitalised for a febrile illness with total WBC counts lower than the normal range for age and sex were at significantly higher risk of dengue. Furthermore, over 85% of hospitalised children with dengue had a WBC count at the time of hospitalisation that was either low or normal and <15% had a high WBC count which is the hallmark of infections in general and bacterial infections in particular.
The mean platelet count at the time of hospitalisation was lower among the hospitalised children with dengue cases when compared with children hospitalised with OFI. However, these differences in the platelet count at the time of hospitalisation were statistically (P = 0.06) not significant [Table 2]. Furthermore, although the mean platelet count among the children with dengue was lower than those among the children with OFI, it was still within the normal range. When the platelet count at the time of hospitalisation among the children with dengue was correlated with their age, there was decline in the count with increasing age and this decline was statistically (P = 0.0015) significant. This finding suggests that lower platelet counts at the time of presentation in dengue are more likely to be present in older children when compared to younger children. When the platelet count was correlated with the duration of the illness, there was no significant change in the count with duration of the illness. When the range of platelet counts was analysed in the two groups, just a little over 25% of hospitalised children with dengue had a platelet count <150 (the lower limit of the normal range) compared with the 10% of children hospitalised with OFI who also had a platelet count at the time of hospitalisation that was below 150 [Table 3]. Studies from Asia have reported a much higher percentage of cases of dengue having platelet counts below the normal range.[10,13,14] This finding of higher platelet counts at the time of presentation among our hospitalised children with dengue compared to those reported from the Southeast Asian countries may be due to less severe form of dengue or due to early presentation for hospitalisation in our settings. In our setting, platelet counts at the time of hospitalisation among children with dengue were normal or higher than the normal range in over 75% of cases and therefore, it is not of much utility in predicting the diagnosis of dengue. Although in several studies from Asia, thrombocytopenia has been reported to have a fair predictive value in predicting the diagnosis of dengue infection.[10,14,15]
CONCLUSION
In summary, febrile children who are confirmed to be dengue are significantly more likely to lower total WBC counts, absolute neutrophil counts and absolute lymphocyte counts as well as the platelet counts at the time of presentation. Lower than normal total WBC counts and lower platelet counts (<150 000) are both associated with a significantly higher risk of dengue. However, this decrease in counts in dengue is not universal and a significant number of febrile children who confirm to be dengue have normal counts at the time of presentation.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Region of the Americas (by Country and Subregion) from 1995 Through 2011. 2007. Pan American Health Organization (PAHO). Available from: http://www.paho.org/english/ad/dpc/cd/dengue.htm [Last accessed on 2011 Feb 04]
- [Google Scholar]
- The epidemiology of dengue in the americas over the last three decades: A worrisome reality. Am J Trop Med Hyg. 2010;82:128-35.
- [CrossRef] [PubMed] [Google Scholar]
- Caribbean Epidemiolgy Center (CAREC) 2009 Available from: http://www.carec.org [Lsat accessed on 2022 Nov 15]
- [Google Scholar]
- Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005;73:1063-70.
- [CrossRef] [PubMed] [Google Scholar]
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control -- New edition 2009-10-01 ed. Geneva: World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases (TDR); 2009. p. :1-147.
- [Google Scholar]
- Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health. 2008;13:1328-40.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and laboratory abnormalities due to dengue in hospitalized children in Mumbai in 2004. Dengue Bull. 2005;29:90-6.
- [Google Scholar]
- Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313-21.
- [CrossRef] [PubMed] [Google Scholar]
- Profile of dengue patients admitted to a tertiary care hospital in Mumbai. Turk J Pediatr. 2011;53:626-31.
- [Google Scholar]
- Caribbean Epidemiology Centre (CAREC/PAHO/WHO) 2008. Available from: http://new.paho.org/carec/dmdocuments/6.%20Dengue%20and%20DHF.pdf [Lsat accessed on 2022 Nov 15]
- [Google Scholar]
- Dengue Virus Infection among Children in Barbados In: Caribbean Health Research Council Annual Conference (53rd ed). 2008.
- [Google Scholar]
- Review of dengue fever cases in Hong Kong during 1998 to 2005. Hong Kong Med J. 2008;14:170-7.
- [Google Scholar]
- Clinical presentations and laboratory findings in suspected cases of dengue virus. Saudi Med J. 2006;27:1711-3.
- [Google Scholar]
- Predictive value of thrombocytopaenia in the diagnosis of dengue infection in outpatient settings. Med J Malaysia. 2007;62:390-3.
- [Google Scholar]







