Translate this page into:
A nuanced presentation of congenital pleural effusion: The chylothorax
*Corresponding author: Sudha Rudrappa, Department of Pediatrics, Kurunji Venkatramana Gowda Medical College and Hospital, Sullia, Karnataka, India. sudharudrappa1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vimal S, Rudrappa S, Sundar SS. A nuanced presentation of congenital pleural effusion: The chylothorax. Karnataka Paediatr J. 2024;39:94-7. doi: 10.25259/KPJ_2_2024
Abstract
This article details a rare instance of congenital pleural effusion, the cause of which was chylothorax. Congenital pleural effusions have several potential causes. To determine the cause, a variety of prenatal and postnatal tests can be performed. The range of presentations and the seriousness of the case’s complications determine the management strategy. A case of chylothorax is presented in this article, which was treated conservatively after postnatal confirmation through pleural fluid analysis.
Keywords
Congenital chylothorax
Pleural effusion
Hydrops
INTRODUCTION
Neonates seldom experience pleural effusions. Both prenatal and postnatal acquisitions are possible. Prenatal pleural effusions have been linked to several conditions, including congenital heart defects, pulmonary malformations, hydrops fetalis, infections contracted in utero, congenital chylothorax and other uncommon infections. Among the most common postnatal causes are iatrogenic ones, such as peritoneal dialysis, central catheter-related complications, infections and newborn tachypnoea that are momentary and traumatic chylothorax.[1]
Prenatal pleural fusions are primarily caused by chylothorax and congenital heart defects. Prenatal pleural effusion most commonly results from congenital chylothorax. It frequently occurs in conjunction with hydrops foetalis and is most likely caused by abnormal lymphatic system development or obstruction.[1]
Antenatally, hydrops foetalis frequently include pleural effusions, which are identified by ultrasonogram (USG). Pulmonary hypoplasia, which can also manifest as severe respiratory distress at birth, can be caused by long-standing effusions that occur before 20 weeks of gestation. Physical findings include diminished breath sounds and dullness to percussion.[2,3]
Neonates who show no symptoms can be carefully treated and frequently observed. Neonates exhibiting symptoms ought to be managed symptomatically, utilising pleural fluid drainage, respiratory supports and empirical antibiotics if required. Pleural fluid should be analysed for all suspected aetiologies. Although serial needle aspirations may not always be successful in removing some of the effusions, tube thoracostomy is usually necessary.[2,3]
In this case study, we present a baby who had an antenatal pleural effusion and, following a thorough evaluation, was found to have chylothorax.
CASE REPORT
A 25-year-old primigravida mother of a non-consanguineous marriage gave birth to a baby boy at 37 weeks and two days through an elective caesarean section.
The mother experienced spontaneous conception, took folic acid supplements regularly and underwent routine prenatal care. The first trimester was uneventful. The nuchal translucency scan showed an increased nuchal fold thickness of 6.7 mm. The mother was treated with diet control after being diagnosed with gestational diabetes mellitus at 22 weeks of pregnancy. At 20 weeks, a level 2 scan revealed a bilateral pleural effusion, right more than left, with cardia displaced to the right side with sufficient foetal growth, and recommended additional testing. No noteworthy results were obtained from the quantitative fluorescent polymerase chain reaction, chromosomal microarray, karyotyping or TORCH panel (toxoplasma gondii, rubella, cytomegalovirus and herpes simplex virus 1 and 2), which was performed through amniocentesis. Antenatal 2 D echo was normal. The term scan also showed minimal bilateral pleural effusion with the right side more than the left side, polyhydramnios and adequate foetal growth [Figures 1 and 2]. Family history was insignificant. The maternal serology (human immunodeficiency virus, hepatitis B surface antigen, hepatitis C virus and venereal disease research laboratory) was non-reactive.
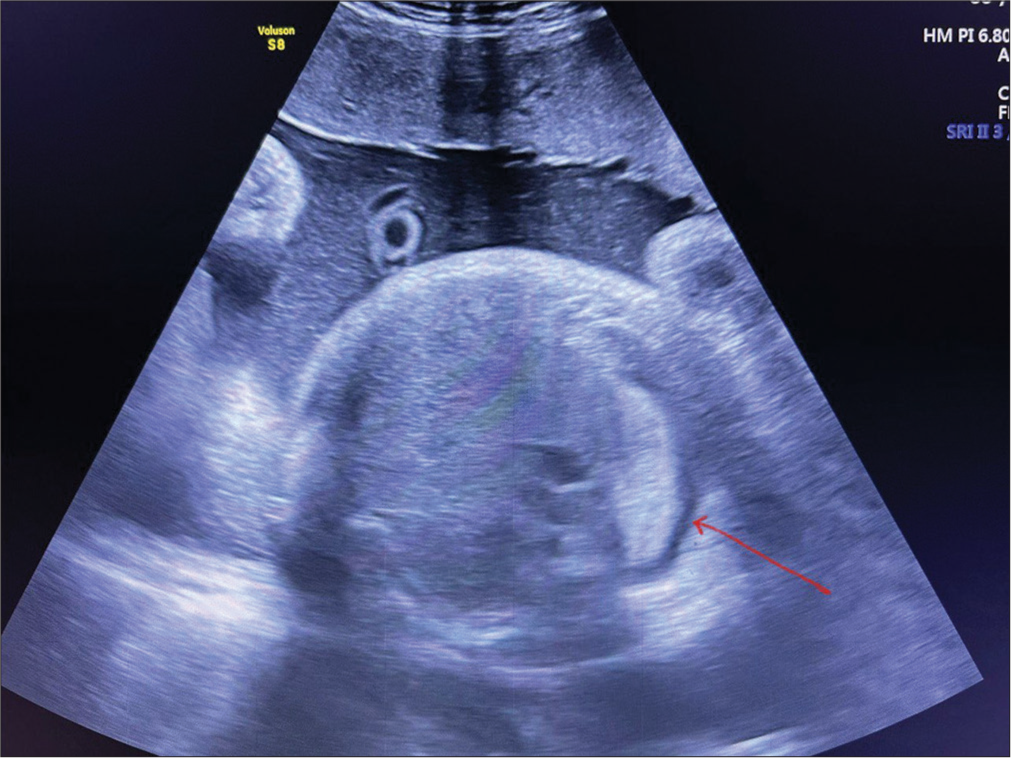
- Antenatal ultrasound scan at 37 weeks showing right-sided pleural effusion (10–15 mL) of pleural fluid (red arrow).
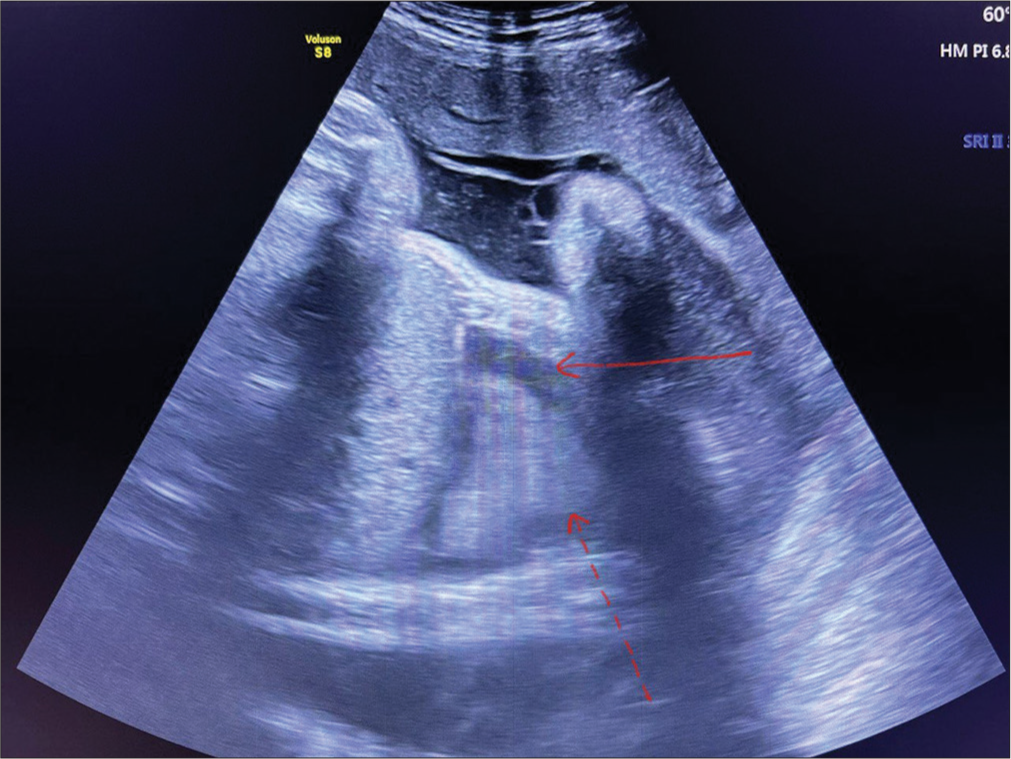
- Antenatal ultrasound scan at 37 weeks showing right-sided pleural effusion (10–15 mL) of pleural fluid (red arrow) and collapsed lung (red dotted arrow).
The baby was delivered through elective caesarean section in view of non-progressive labour. The baby cried immediately after birth. The newborn weighed 3.22 kg, measured 51 cm in length, had a head circumference of 31 cm and a chest circumference of 33 cm. A postnatal scan performed shortly after delivery revealed bilateral pleural effusion. Nothing remarkable was found after a head-to-toe examination. The infant began to exhibit tachypnoea, nasal flaring and mild chest retractions after 30 min of birth. A high-flow nasal cannula (HFNC) ventilation was given for the baby. Arterial blood gas results indicated adequate oxygenation. The results of the USG abdomen and neurosonogram were normal and did not reveal any hydrops-related characteristics. Culture revealed no growth, and the postnatal septic screen was negative. A bilateral pleural effusion was seen on the chest X-ray, and there were no additional contributing findings [Figure 3]. On day 4, HFNC settings were gradually weaned, and in view of unsettling tachypnoea, diagnostic pleural tapping was performed. An examination of the pleural fluid revealed characteristics that pointed to chylothorax– glucose – 53 mg/dL, total protein – 3 g/dL, lactate dehydrogenase – 96 IU/L, total cholesterol 17 mg/dL and triglycerides – 10 mg/dL (before feeding). Over the 8th day, the baby’s tachypnoea improved and was weaned off to room air. Vitamin K was administered along with a birth immunisation. The baby was put on IV fluids at first and then switched to trophic Ryle’s tube feeds, and by day 8, he was being breastfed directly. No signs and symptoms of feed intolerance were noted. Very little pleural effusion and sufficient lower lobe expansion were seen on the pre-discharge USG chest.
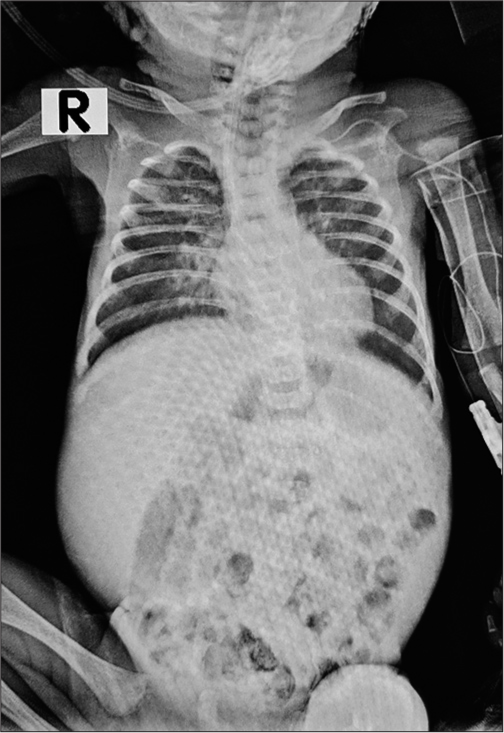
- Frontal chest X-ray, supine film showing mild homogeneous haziness in bilateral lung fields at 2 hours of life.
The prognosis, recurrence, potential complications and consequent requirement for routine follow-up were explained to the patient’s parents.
The baby was followed up after an interval of one month. Repeat chest X-ray and USG of the thorax showed no evidence of fluid collection or recurrence. The baby was healthy, active and feeding well and has received complete vaccination as per schedule, without any evidence of complications. Further, the baby is on a regular 3-month follow-up.
DISCUSSION
Congenital chylothorax is a rare disorder. It is characterised as a non-traumatic pleural effusion that is found 28 days after birth or before birth with a lymphocyte fraction of at least 80%. The term ‘pleural lymph fluid accumulation’ refers to the build-up of lymph that may result from direct lymphatic duct leakage, excessive production or drainage blockage. The incidence of congenital chylothorax is 1/10000–1/24000, with an overall survival rate of 30–70%. Congenital chylothorax may manifest independently or in conjunction with other genetic disorders or syndromes. The genetic syndromes associated with it are Turner syndrome, Noonan syndrome and Down syndrome. The identified congenital abnormalities include pulmonary lymphangiectasia, pulmonary hypoplasia and extrapulmonary lymphatic dysplasia or malformation.[4]
It is possible to detect congenital chylothorax in a foetus or during an early neonatal period. Accumulated pleural fluid in the foetal stage raises intrathoracic pressure, which, in turn, causes the foetal to swallow less amniotic fluid, leading to polyhydramnios and frequently premature labour. It also exacerbates issues that lead to impaired lung development. Prematurity, pulmonary dysplasia and hydrops foetalis are associated with unfavourable outcomes. In addition, pulmonary dysplasia is the most frequent cause of death.[4,5]
An analysis of pleural fluid reveals increased triglycerides, proteins and lymphocyte fractions (>80%). Thoracocentesis, thoracoamniotic shunting and pleurodesis are the available prenatal interventional options. Surgical intervention, nutritional support, drainage of effusions and respiratory support are all part of postpartum care.[6] When treating patients with refractory chylothorax, octreotide administration is recommended. Octreotide can be administered as a continuous intravenous infusion or given twice daily as an intravenous bolus or subcutaneous. The effective daily doses were from 7.2 μg/kg to 240 μg/kg for intravenous infusion. In 3–6 weeks, the majority of newborns experience total remission. Surgical interventions can be attempted, if not within six weeks. The last resort is surgical procedures that involve chemical pleurodesis. There may be a hereditary component to congenital chylothorax, as it is linked to recurrence in later siblings.[7]
CONCLUSION
A full-term baby boy diagnosed with mild antenatal pleural effusion was born. Nothing noteworthy was found during the prenatal workup. Chylothorax was diagnosed based on characteristics found in postnatal pleural fluid analysis that suggested chylous collection. The baby significantly improved after pleural fluid drainage. Any potential complications could be overlooked because there is no long-term follow-up included.
Ethical approval
The research/study complied with the Helsinki declaration of 1964.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Clinical features and outcomes of congenital chylothorax: A single tertiary medical centre experience in China. J Cardiothorac Surg. 2022;17:276.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital chylothorax: Current evidence-based prenatal and post-natal diagnosis and management. Lymphology. 2019;52:108-25.
- [CrossRef] [Google Scholar]
- Congenital chylothorax. Semin Fetal Neonatal Med. 2017;22:234-9.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital chylothorax: A unique presentation of nonimmune hydrops fetalis in a preterm infant. Adv Neonatal Care. 2016;16:114-23.
- [CrossRef] [PubMed] [Google Scholar]
- Le chylothorax de découverte anténatale [Prenatal diagnosis of chylothorax] Arch Pediatr. 1999;6:867-71.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital chylothorax in a late preterm infant and successful treatment with octreotide. Pediatr Neonatol. 2011;52:297-301.
- [CrossRef] [PubMed] [Google Scholar]






