Translate this page into:
Clinical profile of scrub typhus in children at a tertiary care hospital in South India
*Corresponding author: Nadiya Shajahan, Department of Paediatrics, Yenepoya Medical College, Mangalore, Karnataka, India. nadiya209@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shajahan N, Sahana KS. Clinical profile of scrub typhus in children at a tertiary care hospital in South India. Karnataka Pediatr J 2022;37:46-50.
Abstract
Objectives:
The objectives of this study were to study the demographic, clinicolaboratory profile and outcome of scrub typhus in affected children.
Material and Methods:
Retrospective descriptive study of children aged 1 month–16 years who were diagnosed with scrub typhus between January 2014 and November 2021, admitted to the paediatric ward and intensive care unit of our hospital.
Results:
A total of 15 patients were included in the study, of which 60% were male and 40% were female. Among the study population, 66.7% of children were aged above 10 years. The cases were seen mainly in the months of October–November. Fever was present in all children. Other symptoms noted were cough (66.7%), chills and rigours (53.3%), headache (53.3%), abdominal pain (40%) and vomiting (33.3%) and rarely altered sensorium. The diagnostic features such as lymphadenopathy and hepatosplenomegaly were found in 40 and 46.7% of patients, respectively. Laboratory findings noted were elevated CRP (66.7%) and deranged liver enzymes (93.3%). Scrub typhus IgM, Weil-Felix and febrile agglutination test were positive in 33.3%, 53.3% and 26.7% of cases, respectively. Pneumonia (40%), shock (6.7%) and myocarditis (6.7%) were the common complications present in these children.
Conclusion:
Even in the absence of eschar, the diagnosis of scrub typhus should be strongly considered in any febrile child with lymphadenopathy, hepatosplenomegaly, thrombocytopaenia and liver dysfunction.
Keywords
Scrub typhus
Orientia tsutsugamushi
Lymphadenopathy
INTRODUCTION
The obligate intracellular Gram-negative coccobacilli, Orientia tsutsugamushi, a member of the Rickettsiae family, are the cause of acute febrile illness known as scrub typhus. It is also a common tropical disease caused by bite of a chigger, which is a larval stage of the mite Leptotrombidium. Over 1 billion people are at danger worldwide, and an estimated 1 million cases of scrub typhus occur annually. The endemic illness scrub typhus is usually found in the ‘tsutsugamushi triangle’ region of the world. Asia, Australia and Islands in the Indian and Pacific Oceans make up the majority of the region.[1]
The perfect environment for the infected mites to develop may be found in certain sites including grassy regions, riverbanks, tropical rain forests and mountain deserts.[2] Outbreaks of scrub typhus were reported in 2003–2004 and 2007 in Himachal Pradesh, Sikkim and Darjeeling (West Bengal).[3] The number of samples and regions where scrub typhus has been detected has expanded over time. On the other hand, South Indian state, Karnataka has reported a comparatively limited number of paediatric cases of scrub typhus infection.[4]
Delay in diagnosis and initiation of effective therapy might result in significant consequences such as acute respiratory distress syndrome (ARDS), septic shock, multiorgan failure and death. Mortality rates for untreated patients can reach as high as 30–35%.
Due to the non-specific clinical presentation and due to lack of diagnostic facilities, rickettsial illnesses are substantially underdiagnosed in India.[5] The prevalence of scrub typhus in coastal Karnataka is unclear as there are no studies done so far from this region in children. Given the scarcity of data available on paediatric scrub typhus, we undertook a retrospective study in a teaching hospital in coastal Karnataka, with an objective to determine the demographic and clinical-laboratory profile including the outcomes of scrub typhus infection in children.
MATERIAL AND METHODS
This is a retrospective descriptive study of all children diagnosed with scrub typhus admitted to the paediatric ward as well as the intensive care unit of our hospital. All children aged 1 month–16 years who tested positive for scrub typhus using either the IgM enzyme-linked immunosorbent assay (ELISA) (InBios Scrub Typhus Detect IgM ELISA kit) or the Weil-Felix test (OX K titre >1:160) or the febrile agglutination test (FAT) from January 2014 to November 2021 were included in the study. Children with other coinfections, such as dengue, malaria, typhoid and leptospirosis confirmed by appropriate laboratory tests were excluded from the study. After seeking ethical clearance from the Institutional Ethics Committee, the study was conducted for 2 months. The list of patients diagnosed with scrub typhus over the past 7 years within the paediatric age group was obtained from the medical records department. We collected data of demographical details, clinical details, management and complications in this retrospective descriptive study, and the same information was recorded on a standardised study pro forma.
Statistical methods
For quantitative variables, descriptive analysis was performed using the mean and standard deviation, and for categorical variables, descriptive analysis was performed using frequency and percentage. The data were then analysed using IBM SPSS software version 22 for statistical analysis.
RESULTS
During the study period, 15 children were diagnosed with scrub typhus infection. There were nine males and six females in all. Children as young as 1 month and as old as 16 years were included in the study, with a mean age of presentation of 6 years. [Table 1] represents the sociodemographic profile of the patients. According to [Figure 1], the months of October– November recorded the highest number of positive cases. This was right at the end of rainy season, which made the conditions ideal for mites. The majority of the cases that were reported at our institution were from Central Karnataka, with the exception of one case that originated in the Malenadu region.
| Data | No. (%) |
|---|---|
| Gender | |
| Male | 9 (60) |
| Female | 6 (40) |
| Age distribution | |
| 1 month–5 years | 1 (6.7) |
| 6–10 years | 4 (26.7) |
| 11–16 years | 10 (66.7) |
| Residence | |
| Central Karnataka | 10 (66.6) |
| Coastal Karnataka | 2 (13.3) |
| North Karnataka | 3 (20) |
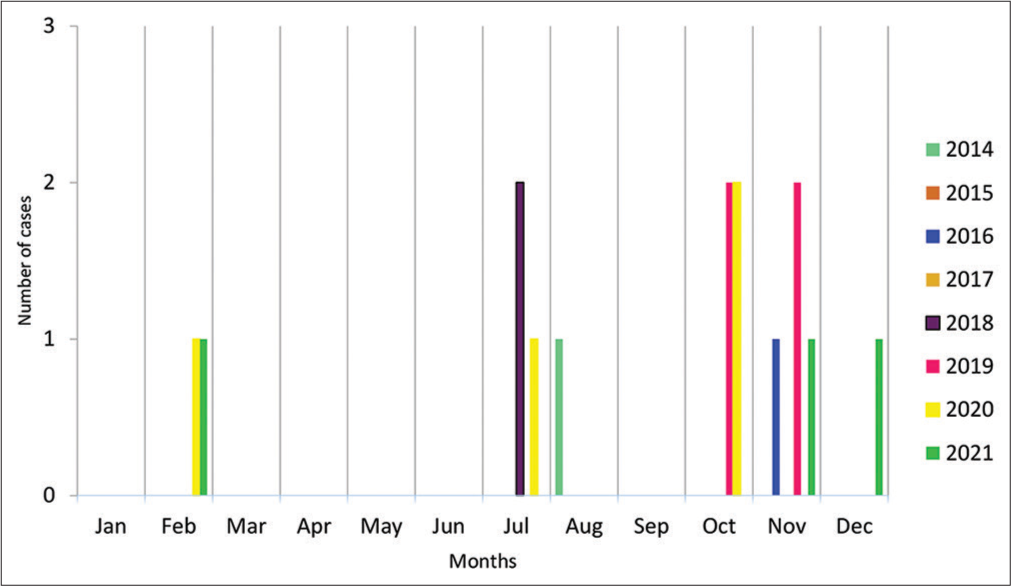
- Month-wise distribution of scrub typhus cases during the study period (January 2014–November 2021).
Fever was noted in all 15 cases. Twelve individuals presented with a high-grade fever (temperature> 100.5°F) and three with a low-grade fever (temperature <100.4°F), of which 11 individuals had intermittent fever and four had continuous fever. Cough (66.7% of patients), chills and rigours (53.3%), headache (53.3%), abdominal pain (40%) and vomiting (33.3%) were the other significant clinical symptoms. Hepatosplenomegaly and lymphadenopathy were observed in 46.7% and 40% of children, respectively. Skin rash (maculopapular and erythematous) involving trunks and extremities excluding palms and soles were observed in three cases. None of the children had vasculitis type of rash. Eschar was not present in any of the children in our study. [Table 2] provides further information on the clinical profile.
| Symptoms | No. (%) | Examination findings | No. (%) |
|---|---|---|---|
| Fever | 15 (100) | Pallor | 10 (66.7) |
| Duration of fever | Hepatosplenomegaly | 7 (46.7) | |
| 1–3 days | 3 (20) | Lymphadenopathy | 6 (40) |
| 4–7 days | 1 (6.7) | Tachypnoea | 6 (40) |
| >7 days | 11 (73.3) | Throat congestion | 4 (26.7) |
| Cough | 10 (66.7) | Splenomegaly | 3 (20) |
| Chills and rigours | 8 (53.3) | Ascites | 3 (20) |
| Headache | 8 (53.3) | Pleural effusion | 3 (20) |
| Abdominal pain | 6 (40) | Shock | 2 (13.3) |
| Vomiting | 5 (33.3) | ||
| Breathing difficulty | 4 (26.7) | ||
| Rashes | 3 (20) | ||
| Oedema | 3 (20) | ||
| Bleeding | 2 (13.3) | ||
| Altered sensorium | 1 (6.7) | ||
| Seizures | 1 (6.7) |
Significant abnormal laboratory findings noted were anaemia (66.7%), elevated CRP (66.7%), thrombocytopaenia (33.3%), deranged liver enzymes (93.3%) and albuminuria (60%) [Table 3]. Scrub typhus IgM, Weil-Felix and FAT were positive in 33.3%, 53.3% and 26.7% of cases, respectively.
| Laboratory findings | No (%) |
|---|---|
| Anaemia | 10 (66.7) |
| Leukopenia | 2 (13.3) |
| Thrombocytopaenia | 5 (33.3) |
| Elevated CRP | 10 (66.7) |
| Increased S. creatinine | 2 (13.3) |
| S. bilirubin >1.2mg% | 4 (26.7) |
| SGOT >40 | 14 (93.3) |
| SGPT >40 | 10 (66.7) |
| Hypoalbuminaemia | 8 (53.3) |
| Albuminuria | 9 (60) |
| Hyponatraemia | 6 (40) |
| Deranged INR | 4 (26.7) |
| Scrub typhus IgM | 5 (33.3) |
| Weil-Felix | 8 (53.3) |
| FAT | 4 (26.7) |
FAT: Febrile agglutination test
The most common ultrasonographic findings observed in our study were splenomegaly (26.7%), hepatosplenomegaly (13.3%) and polyserositis (20%). Abnormal chest radiographic features such as pleural effusion and pneumonia were observed in 20% and 13.3% of the individuals.
The therapeutic modalities that were used most frequently in our study were IV antibiotics, IV fluids and oxygen support. Doxycycline, the medication of choice for scrub typhus, was administered to all patients who had been diagnosed with the disease. Children who were in critical condition were treated with azithromycin. About 13.3% of children received inotropes. Blood products (PRBC, platelets and FFP) were administered to 40%, 20% and 6.7% of children, respectively.
Among the 15 cases, six children developed complications. Pneumonia was the most common complication observed (40 %). Acute kidney injury (AKI), shock, DIC, encephalitis, myocarditis, heart failure, sepsis and multiple organ dysfunction syndrome (MODS) were observed in 6.7% of the individuals. All the 15 individuals survived. The average length of hospital stay was 7 days. One patient had the longest stay, which lasted for a total duration of 25 days.
DISCUSSION
Scrub typhus is one of the neglected and most common re-emerging illnesses. The prevalence of scrub typhus has been reported from several part of India by Mittal et al., Gurung et al., Gupta et al.[6-8] and others. We observed a high prevalence of cases in Central Karnataka, since our hospital is a referral centre from Central Karnataka.
In this study, we noticed that the months of October– December had a higher incidence of scrub typhus, and the bulk of cases were recorded in 2019 and 2020. The peak number of cases, as noted by Varghese et al., occurred between September and February, which are thought to be cooler months. However, Weitzel et al., in a study conducted in South America, found that all cases of scrub typhus occurred between January and February, indicating that the summer may be the period of vector activity.[9] The epidemiology revealed a male predominance in our study. However, Weitzel et al. and Varghese et al. found female predominance in their study over 50 years of age.[9,10]
The incidence rate was greater among those between the ages of 11 and 16, which may be probably due to their more engagement in outdoor activities compared to the younger ones. Bhat et al. also observed in their study that two-thirds of cases included children between the ages of 12 and 18, with only one-third under the age of 12.[11]
Fever was the most common presenting symptom (100%), followed by cough (66.7%), chills and rigours (53.3%) and headache (53.3%), similar to the study from South India. Oedema was observed in 20% of patients, which is equivalent to the study from Northeast India. The most common signs reported were pallor (66.7%) followed by lymphadenopathy (40%) and hepatosplenomegaly (46.7%). Incidence of splenomegaly was equivalent to that of the previous studies, although hepatomegaly was lower. The analysis of clinical examination indicates that the symptoms and signs were non-specific, which may be one of the causes for delayed diagnosis.[12]
Common laboratory findings were deranged liver enzymes (93.3%), anaemia (66.7%) and elevated CRP (66.7%). Palanivel et al.[13] also observed elevated liver enzymes in 64% of cases. Thrombocytopaenia was seen in 33.3% of cases whereas Murali et al.[14] noted thrombocytopaenia in more than 50% of children.
The most common complication observed in our study was pneumonia (40%), followed by AKI, shock, DIC, encephalitis, myocarditis, heart failure, sepsis and MODS in 6.7% of individuals. Similar findings from a research by Palanivel et al. also stated that shock, ARDS, acute renal failure, MODS and DIC were the main causes of mortality. One of the factors contributing to a poor outcome was the late referral to hospital with organ dysfunction.
IV antibiotics were used in 80% of cases of our study population. Children received doxycycline for a total duration of 7 days, whereas severe and complicated cases required 10 days therapy. Doxycycline was utilised in 85.5% and azithromycin in 14.5% of the children in a study by Madhusmita et al.,[15] while chloramphenicol was also used in a study by Dass et al.[16] As per the records, the average time taken for resolution of fever after initiation of treatment was 2 days in our study, as opposed to 2.4 days in a study from the Northeast and 2.8 days reported by Huang et al.[17] Doxycycline is the most appropriate and cost-effective antibiotic against scrub typhus.
CONCLUSION
A frequent cause of pyrexia that lacks a clear source could be scrub typhus and its clinical symptoms and signs are comparable to many tropical infectious diseases. Eschar, the most significant clinical sign, is often overlooked. Even in the absence of eschar, the diagnosis of scrub typhus should be strongly considered in any febrile child with lymphadenopathy, hepatosplenomegaly, thrombocytopaenia and liver dysfunction.
Acknowledgement
The authors thank Institutional Ethical Committee board for approval and permission to publish this manuscript.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Molecular epidemiology and genetic diversity of Orientia tsutsugamushi from patients with scrub typhus in 3 regions of India. Emerg Infect Dis. 2015;21:64-9.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus: The geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48:S203-30.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and seroimmunological profile of scrub typhus in Bengaluru, Southern India. J Clin Diagn Res. 2020;14:32-6.
- [CrossRef] [Google Scholar]
- Scrub typhus as a cause of acute encephalitis syndrome, Gorakhpur, Uttar Pradesh, India. Emerg Infect Dis. 2017;23:1414-6.
- [CrossRef] [PubMed] [Google Scholar]
- Outbreak of scrub typhus in the North East Himalayan region-Sikkim: An emerging threat. Indian J Med Microbiol. 2013;31:72-4.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of cutoff of ELISA and immunofluorescence assay for scrub typhus. J Glob Infect Dis. 2016;8:97-9.
- [CrossRef] [PubMed] [Google Scholar]
- Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-61.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and risk factors of scrub typhus in South India. Indian J Med Res. 2016;144:76-81.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus in children at a tertiary hospital in North India: Clinical profile and complications. Iran J Pediatr. 2014;24:387-92.
- [Google Scholar]
- Acute encephalitis syndrome and scrub typhus in India. Emerg Infect Dis. 2017;23:1434.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile of scrub typhus in children. Indian J Pediatr. 2012;79:1459-62.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical, laboratory profile and outcome of scrub typhus in children. IOSR J Dental Med Sci. 2016;15:30-2.
- [Google Scholar]
- Profile of pediatric scrub typhus in Odisha, India. Indian pediatr. 2019;56:304-6.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics of pediatric scub typhus during an outbreak in the North Eastern region of India: Peculiarities in clinical presentation, laboratory findings, and complications. Indian J Pediatr. 2011;78:1365-70.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus in children in a teaching hospital in Eastern Taiwan, 2000-2005. Southeast Asian J Trop Med Public Health. 2009;40:789-94.
- [Google Scholar]






