Translate this page into:
Hepatitis-associated aplastic anaemia – A rare case presentation with literature
*Corresponding author: Kingsley Onorhide Akaba, Department of Haematology and Blood Transfusion, University of Calabar Teaching Hospital, Calabar, Nigeria. akaba_kingsley@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Akaba KO. Hepatitis-associated aplastic anaemia – A rare case presentation with literature. Karnataka Paediatr J. 2024;39:1-4. doi: 10.25259/KPJ_32_2024
Abstract
Hepatitis-associated aplastic anaemia is a rare disorder that constitutes both diagnostic and management dilemma, especially in low-resource settings and it is underreported or misdiagnosed when compared to other causes of aplastic anaemia in children. In this research, we describe a 14-year-old Nigerian male who presented with seronegative hepatitis of unknown origin and subsequently developed aplastic during hospitalisation. Aplastic anaemia associated with hepatitis is a rare but potentially lethal condition that is overlooked or inadequately reported. Our patient illustrates that hepatitis-associated aplastic anaemia is immune-mediated aplastic anaemia. Furthermore, as far as we are concerned, this represents the fundamental study of its kind in our region. Consequently, we advise healthcare professionals to remain vigilant for cases of hepatitis that exhibit characteristic pancytopenia and conduct an investigation to facilitate early diagnosis of possible aplastic anaemia.
Keywords
Hepatitis
Associate
Aplastic anaemia
INTRODUCTION
Aplastic anaemia is an unconventional and potentially life-threatening condition characterised by chronic primary failure of haematopoiesis resulting in a reduction or absence of haematopoietic precursors in the bone marrow accompanied by pancytopenia. This condition can either be hereditary or acquired.[1] The non-hereditary form presents with decreased cellularity and replacement of bone marrow with fatty tissue. The exact aetiology remains largely unknown, but idiopathic cases account for approximately 65% of cases. Fanconi anaemia represents the most common hereditary cause. In addition, seronegative hepatitis is implicated in 5–10% of all cases, while 5–10% of adult-onset aplastic anaemia is linked to telomerase defect.[1] Other contributing factors may include abnormal immune responses triggered by viruses, chemical exposure and certain medications. Hepatitis-associated aplastic anaemia (HAAA) is a type of acquired aplastic anaemia where a shrewd hepatitis infection precipitates failure of the bone marrow and myelosuppression. This condition was first documented by Lorenz and Quaiser in 1955.[2,3] HAAA is relatively new and occurs more frequently in the East compared to the West[3], and individuals from economically disadvantaged settings are more affected. They typically manifest with a rapid decrease in all the cell lines. While the disease is primarily associated with viruses, such as cytomegalovirus (CMV), parvovirus B19 Epstein–Barr virus (EBV), hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV) and echovirus, understanding of its pathogenesis remains elusive.[4] HAAA is not dependent on clinicodemographic such as age, sex and severity of hepatitis, but predominantly in adolescent males and children.[5] HAAA is classified either into self-limiting or fulminant, acute or chronic and mild or transient.[5] Its pathogenesis remains a mystery; however, several studies have shown it to be an immune-mediated couple to the development of HAAA and response to immunosuppressive therapy (IST), haematopoietic stem cell transplantation and liver transplantation in children with non-A, non-B and non-C hepatitis-related failure serve as a revitalising management of HAAA. Thus, this report and literature review aims to report the first case of HAAA with diagnostic and management dilemmas in a resource-constrained environment like ours.
CASE REPORT
A 14-year-old male from Nigeria visited the emergency unit of the paediatric department of the University of Calabar Teaching Hospital with a complaint of persistent jaundice and petechiae on the upper and middle 1/3 of his right thigh. In addition, his mother mentioned that a week ago, he had nosebleeds. Jaundice is reported to have occurred a month before admission. Laboratory evaluations indicate that liver enzymes were markedly elevated. Based on these abnormalities, a diagnosis of hepatitis was queried. We conducted a complete blood count and liver function tests, both of which were found to be abnormal. The results for the liver function tests, clotting profile and complete blood counts are presented in Tables 1-3 below. On account of the impaired liver function, the patient was managed as a case of acute hepatitis. On our primary physical examination, his sclera appeared icteric. He also had evidence of ecchymosis on his right thigh.
| Date | ALT (5–40) IU/L | AST (5–40) IU/L | ALP (22–160) IU/L | GGT (10.0–71.0) U/L | TSB (2–17) umol/L | DSB (2–7) umol/L | Labs |
|---|---|---|---|---|---|---|---|
| August 7, 24 | 488 | 538 | 90 | 170 | 118 | 92 | Nosam |
| August 13, 24 | 74 | 57 | 1086.8 | 74.8 | 38.4 | Arubah | |
| August 19, 24 | 440 | 205 | 76 | 67 | 44 | 23 | Nosam |
| September 3, 24 | 123 | 254 | 69 | 107 | 40 | 25 | Nosam |
ALT: Alanine transaminase, AST: Aspartate transaminase, ALP: Alkaline phosphatase, GGT: Gamma-glutamyl transferase, TSB: Total serum bilirubin, DSB: Direct serum bilirubin
| Date | APTT (30–40) s | APTT control | PROTHR time | PROTHR control | ISI | INR (2.0–4.0) | PROTHR ratio |
|---|---|---|---|---|---|---|---|
| August 7, 24 | 32.4 | 27.7 | 11.3 | 12.6 | 1.25 | 0.87 | 0.90 |
| August 20, 24 | 32.1 | 27.7 | 12.8 | 12.5 | 1.14 | 1.03 | 1.02 |
APTT: Activated partial thromboplastin time, PTOTHR: Prothrombin time, ISI: International sensitivity index, INR: International normalized ratio
| Date | PCV % | HB g/dL | TWBC | NEUT | LYMPH | PLT | Labs |
|---|---|---|---|---|---|---|---|
| August 7, 24 | 21.1 | 7.3 | 0.8 | - | - | 65,000 | Nosam |
| August 16, 24 | 17.5 | 5.9 | 0.4 | - | - | 28,000 | Nosam |
| August 19, 24 | 20.1 | 6.8 | 0.4 | - | - | 26,000 | Nosam |
| August 24, 24 | 17.4 | 5.6 | 0.34 | - | - | 11,000 | |
| September 3, 24 | 18.3 | 6.4 | 0.3 | - | - | 24,000 | Nosam |
| September 7, 24 | 15.0 | 4.9 | 0.4 | - | - | 9,000 | Nosam |
| September 9, 24 | 16.6 | 5.0 | 0.6 | 64 | 30 | 69,000 | Asi ukpo |
PCV:Packed cell volume, HB:Haemoglobin, TWBC:Total white blood cell counts, NEUT:Neutrophil count, Lymph:Lymphocyte counts, PLT:Platelet count
CBC revealed pancytopenia as shown below:
Reticulocyte count - 0.2%
Stool microscopy done on August 16, 2024
Macroscopy – brownish formed stool, no mucus or blood seen
Microscopy – No ova or cyst of parasite seen
Culture – No stool pathogen isolated after 5 days of incubation.
In our quest to determine the aetiology of hepatitis, we conducted tests for the following viral hepatitis agents, which include hepatitis virus A, B and C; furthermore, CMV and EBV were also investigated, and all came negative. We also investigated for anti-liver, kidney microsomal antibody (LKM) antibody (Ab), anti-double stranded DNA (anti-dsDNA) Ab, cytoplasmic antineutrophil cytoplasmic antibody (cANCA) and perinuclear antineutrophil cytoplasmic antibody (pANCA) to rule out the possibility of autoimmune disorders, none were found to be positive. We also asked about his past drug history (including herbal drugs) and any potential toxin exposure, which were all negative anaemia. Bone marrow aspiration and biopsy were done due to the pancytopenia. The results revealed severe hypocellularity (approximately 10%), which was low for his age and suggestive of aplastic anaemia. Pictures of the bone marrow biopsy and aspiration are shown in Figures 1-3 below.
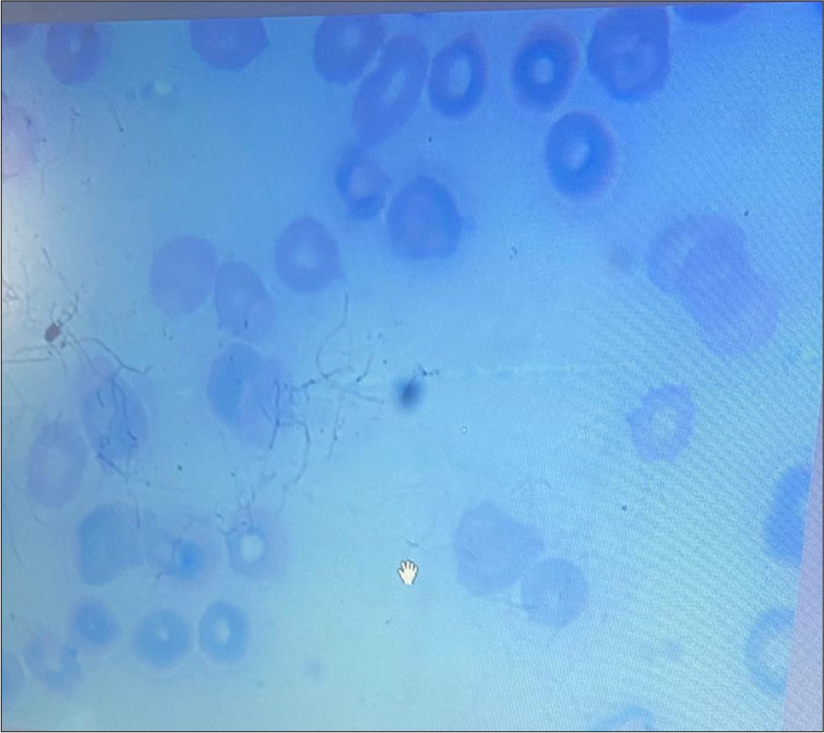
- Peripheral film showing pancytopenia. (Giemsa Stain, x100).
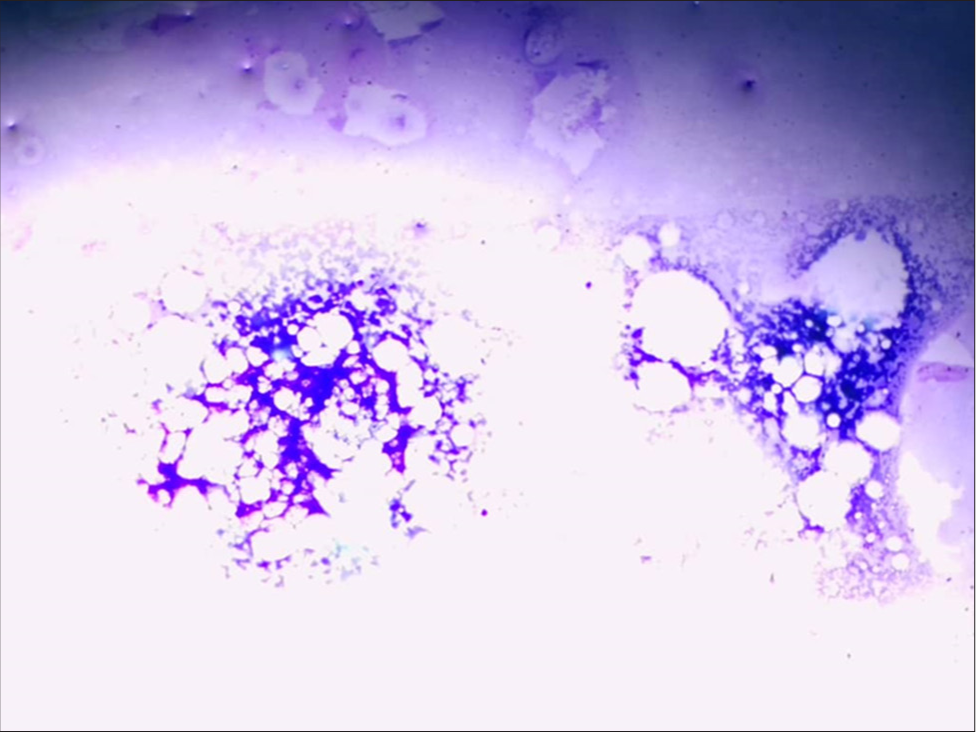
- Bone marrow showing hypocellularity. (Giemsa Stain magnification x10).
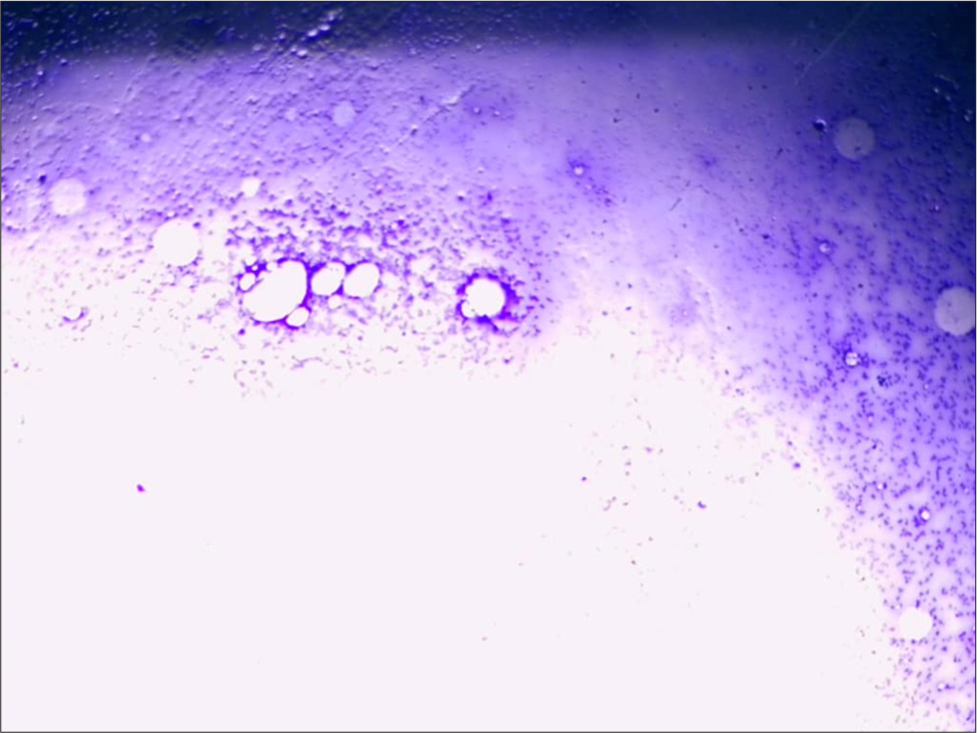
- Bone marrow showing hypocellularity. (Giemsa Stain, Magnification x10).
However, immunophenotyping through flow cytometry was not done on his bone marrow sample to exclude leukaemia or lymphoma due to financial constraints. Our patient was commenced on supportive therapy erythropoietin and filgrastim with no avail; premise on this patient was commenced on whole blood transfusion and platelet concentrate. On receiving the bone marrow report, the patient was commenced on IST, equine antithymocyte globulin at a dosage of 40 mg/kg/day for 4 days, along with prednisolone at 0.5 mg/kg/day, and cyclosporine 10 mg/kg/day. Following this treatment, his CBC stabilised, allowing for discharge from the hospital, with a follow-up appointment scheduled 10 days after initiation of IST. Ultimately, the patient was discharged in a stable state.
DISCUSSION
Hepatitis-associated aplastic anaemia is a critical condition that predominantly affects adolescents and young children, with a higher incidence in males. It necessitates thorough evaluation and management. Notably, serological and virological parameters for hepatitis A, B and C were found negative in the majority of reported cases.[6] This calls for serious concern, especially in low-resource settings like ours, with the challenge of molecular diagnosis. More so, the awareness is poor due to the rarity of HAAA and the seronegativity of most cases, as in the index patient, who was said to be negative to all the serotypes of HAV, HBV, HCV and hepatitis E virus. Also, there was a derangement of the liver enzymes as documented in most literature preceding the pancytopenia, which progressive or acutely leads to aplastic anaemia.[7] The index case peripheral blood film showed pancytopenia, and the bone marrow was severely hypocellular for age, and a diagnosis of aplastic anaemia was made about 2 months before the onset of jaundice. This is similar to the finding by Gonzalez-Casas et al.[8] where they reported that severe aplastic anaemia usually occurs between 2 and 3 months after acute hepatitis. On account of the above diagnosis, we started IST, which is made up of anti-thyroglobulin, cyclosporine and steroids. Similar studies also reported a 30–70% response rate of children with HAAA to IST.[8,9] Our index patient was commenced on a comparable regimen , was given blood products as the need arose, and after 2 weeks, we noticed that our patient’s CBC was stable on follow-up. However, we are still looking for any other superior regimen till we are able to provide him a definitive treatment, Bone marrow transplant.
CONCLUSION
Hepatitis-associated aplastic anaemia is a rare yet potentially fatal condition that is understated and overlooked. Our case study shows that HAAA is immune-mediated aplastic anaemia. Furthermore, as far as we know, this seems to be a pioneer study of its kind in our region. Consequently, we advise vigilance in monitoring cases of hepatitis that exhibit clinical features of pancytopenia, along with conducting thorough follow-ups to facilitate early diagnosis for possible aplastic anaemia.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- UK Health Security Agency. 2022. Available from: https://www.gov.uk/government/news/increase-in-hepatitis-liver-inflammation-cases-in-children-under-investigation#full-publication-update-history [Last accessed on 2024 Sep 22]
- [Google Scholar]
- Multi-country-acute, severe hepatitis of unknown origin in children. 2022. WHO. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON376 [Last accessed on 2024 Sep 22]
- [Google Scholar]
- The diagnosis and treatment of aplastic anemia: A review. Int J Hematol. 2015;101:527-35.
- [CrossRef] [PubMed] [Google Scholar]
- Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-19.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatitis-associated aplastic anemia in pediatric patients: Single center experience. Transfus Apheres Sci. 2020;59:102900.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review: Hepatitis-associated aplastic anaemia-a syndrome associated with abnormal immunological function. Aliment Pharmacol Ther. 2009;30:436-43.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatitis associated aplastic anemia: A review. Virol J. 2011;8:87.
- [CrossRef] [PubMed] [Google Scholar]






