Translate this page into:
Influenza B-associated acute necrotising encephalopathy of childhood treated with tocilizumab
*Corresponding author: Rashmi Adiga, Department of Pediatric Neurology, Rainbow Children’s Hospital, Bengaluru, Karnataka, India. rashmiadiga26@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Adiga R, Sinha S, Vemgal P, Jaiswal A, Kadri A, Krishnamurthy A. Influenza B-associated acute necrotising encephalopathy of childhood treated with tocilizumab. Karnataka Paediatr J. doi: 10.25259/KPJ_12_2025
Abstract
The objective of the study is to describe the clinical presentation, investigations and management of a child with severe ANEC treated with tocilizumab in combination with methylprednisolone (MTP) and intravenous immunoglobulins (IVIG) with good outcome. Review of case notes and literature was done. A 10-year-old previously fit and well girl presented with fever, vomiting, loose stools with severe dehydration and shock. She had one episode of generalised-tonic-clonic-seizure, following which she had a reduced level of consciousness. She was invasively ventilated and received paediatric intensive care unit care. Neuroprotection given until day 3. Levetiracetam, oseltamivir, antibiotics and vitamins were started. Magnetic resonance imaging brain revealed a trilaminar pattern of diffusion-restriction in bilateral thalami on apparent diffusion coefficient (ADC) images and T2 and fluid-attenuated inversion recovery hyperintense areas in the brainstem, bilateral cerebellar hemisphere and thalami with diffusion-restriction suggestive of ANEC. Cerebrospinal fluid (CSF) analysis showed elevated protein, normal glucose, and no cells; the herpes simplex virus-polymerase chain reaction was negative. Electroencephalogram showed abnormal background with slowing, suggesting encephalopathy with no epileptiform activity. ANEC severity score was 8/9 (shock-3, brainstem lesions-2, age-2, CSF Protein-300 mg/dL-1). Hence, she was given MTP 30 mg/kg, a single dose of tocilizumab 800 mg and IVIG at 2 g/kg. The serum interleukin-6 level was elevated. Flu-panel showed influenza B. She was extubated on day 12. She was discharged home on day 19 and made a neurological recovery with supportive therapies and steroids. At present, a year later, the child is well except for mild squint and mild coordination difficulty and is attending mainstream school.
Keywords
Acute necrotising encephalopathy of childhood (ANEC)
Acute necrotising encephalopathy of childhood severity score
Methylprednisolone
Tocilizumab
INTRODUCTION
Acute necrotising encephalopathy of childhood (ANEC) is a rare clinico-radiological syndrome with alteration of consciousness and distinct bilateral symmetric brain lesions mainly involving the thalamus. Studies have described a high mortality rate and severe neurological sequelae.
CASE REPORT
A 10-year-old girl presented with low-grade fever, vomiting, loose stools and poor oral intake. On arrival at the emergency room, she was noted to have severe dehydration and shock. Her Glasgow Coma Scale was 11/15 at the time of presentation. She was treated with fluid boluses and started on ceftriaxone, metronidazole, acyclovir and oseltamivir.
Initial blood tests showed elevated C-reactive protein (28 mg/L) with neutrophilic leucocytosis (24030 cells/mm3), elevated creatinine level (1.8) and elevated liver enzymes with normal serum electrolytes. Dengue rapid and typhoid rapid were negative.
A few hours after admission she had one episode of generalised-tonic-clonic seizure, which stopped with intravenous lorazepam. Following this, she had a reduced level of consciousness.
In view of reducing the level of consciousness, she was intubated and ventilated and received Paediatric Intensive Care Unit care. Levetiracetam was started. Neuroprotection measures were started.
Electroencephalogram (EEG): Background was abnormal, with slowing suggesting encephalopathy. There was no evidence of any epileptiform activity [Figure 1].
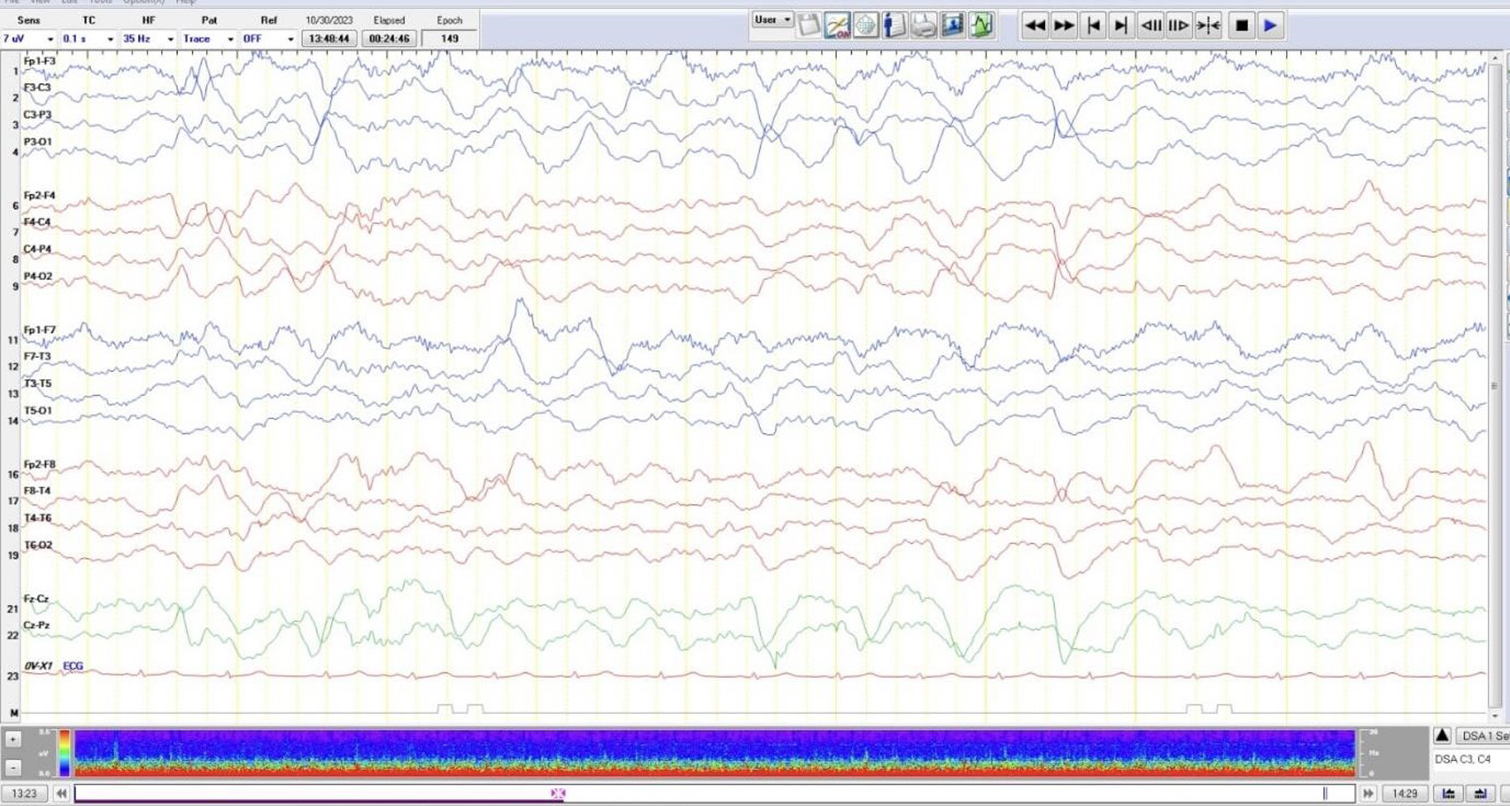
- Electroencephalogram: Background was abnormal with slowing suggesting encephalopathy. There was no evidence of any epileptiform activity.
Magnetic resonance imaging (MRI) brain revealed a trilaminar pattern of diffusion restriction in bilateral thalami on ADC images and T2 and fluid-attenuated inversion recovery hyperintense areas in the brainstem, bilateral cerebellar hemisphere and thalami with diffusion-restriction suggestive of acute necrotising encephalopathy of childhood (ANEC) [Figures 2-5].
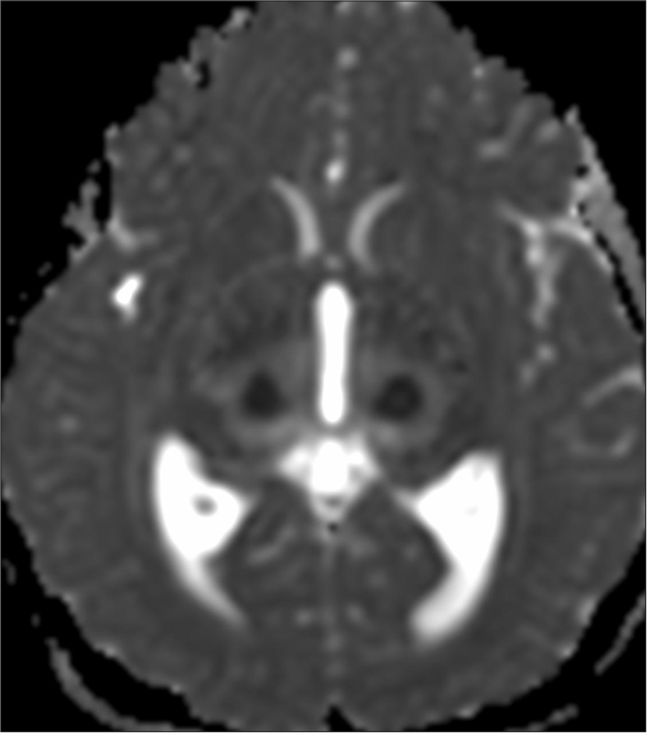
- Magnetic resonance imaging brain: Apparent diffusion coefficient images showing trilaminar pattern of diffusion restriction in bilateral thalami.
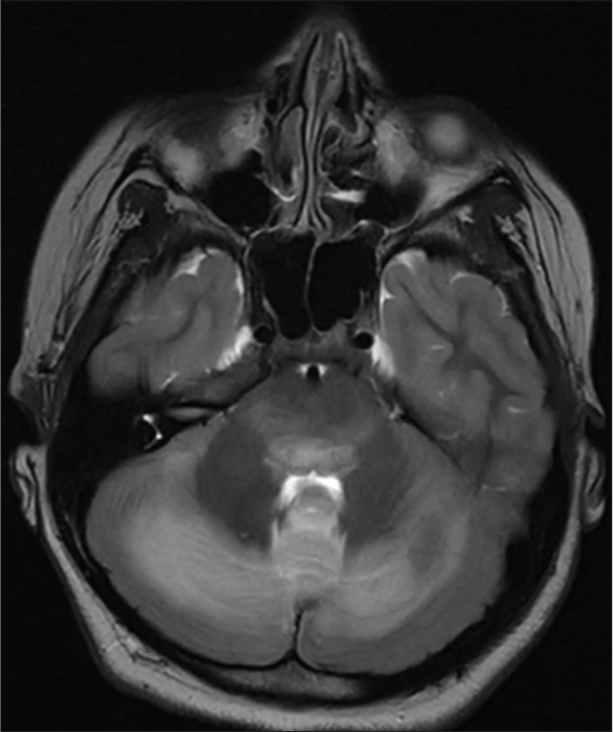
- Magnetic resonance imaging brain T2 hyperintense areas in brainstem.
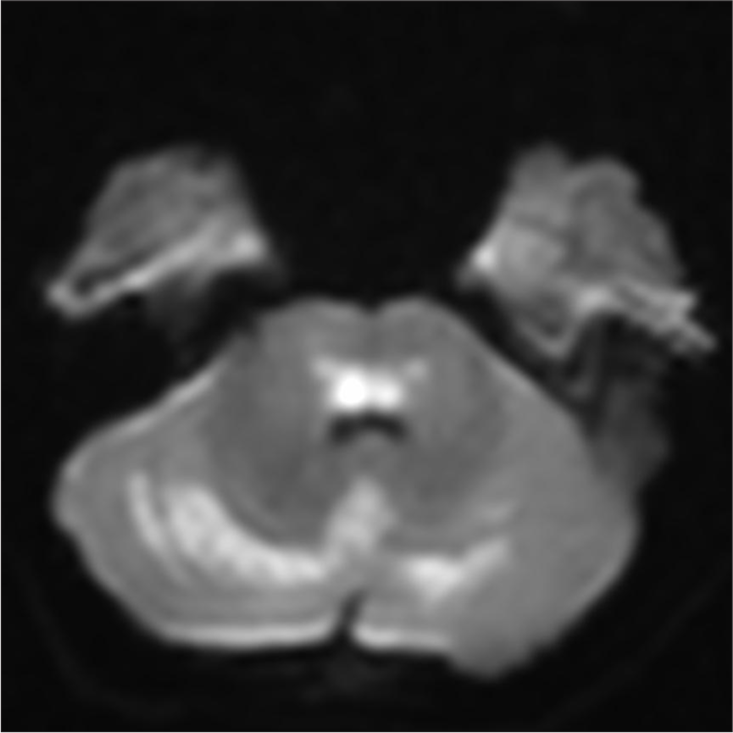
- Brain T2 hyperintense areas in bilateral cerebellar hemisphere showing diffusion restriction.

- Magnetic resonance imaging brain: T2 hyperintense areas in thalami.
Cerebrospinal fluid (CSF) analysis showed elevated protein levels 310 mg/dL and normal glucose 73 mg/dL with no cells.
In view of MRI imaging suggesting ANEC, the child was given an intravenous pulse dose of methylprednisolone (MTP) 30 mg/kg which was given for 5 days and the dose was tapered every 7 days. She was also given a single dose of tocilizumab 800 mg. On day 2 of admission, she was also given intravenous immunoglobulin (IVIG) 2 g/kg over 24 h. She was also started on injection, thiamine and B vitamins and tablet biotin, tablet co-enzyme Q, tablet L carnitine and tablet riboflavin through a nasogastric tube (Mito-cocktail).
Acyclovir was stopped as the herpes simplex virus-polymerase chain in CSF was negative. CSF-IL 6 level was elevated (82.6 pg/mL). The nasopharyngeal swab was positive for influenza B.
Since she had spontaneous respiratory effort, she was given a trial of extubation on day 5; however, in view of increased respiratory distress and poor sensorium, she was reintubated. On day 8, she had minimal pleural effusion which was treated with intravenous furosemide followed by oral diuretics.
She gradually improved and was extubated on day 10 of admission. Repeat interleukin (IL)-6 levels showed a reducing trend (40.10 pg/mL).
Her sensorium gradually improved and she was discharged home on day 19 on oral steroids, levetiracetam and vitamins (mito-cocktail). She was on nasogastric tube feeds but was able to swallow liquids. At discharge, she could sit with support, had antigravity movements of the upper and lower limbs, understood verbal commands and followed the same but was unable to vocalise.
She was started on intensive outpatient neurorehabilitation with physiotherapy, occupational therapy and speech therapy. 6 weeks into the illness, she was able to walk independently and started to read well. 3 months later, levetiracetam was stopped following a normal EEG. Vitamin supplements were stopped after 6 months.
At present, a year later, she is well except for mild squint and mild coordination difficulty and is attending mainstream school.
DISCUSSION
ANEC is an acute neuroinflammatory condition characterised by selective, symmetric brain lesions affecting the bilateral thalami with or without other brain involvement.[1] According to Mizuguchi et al., 40% of the patients had convulsions, 28% had decreased consciousness and 20% had vomiting.[2] This child presented with high-grade fever, loose stool, vomiting, seizure episodes and poor sensorium.
Although the exact pathogenesis of ANEC remains obscure, it is generally considered to be a parainfectious disease triggered mainly by viral infections, influenza virus and human herpesvirus 6 being the two most common types.[3] In our case, influenza B was positive.
The diagnosis of ANEC was determined by specific diagnostic criteria as described by Mizuguchia, which consist of
Encephalopathy preceded by viral febrile illness with rapid deterioration in the level of consciousness and convulsions.
Absent CSF pleocytosis.
Symmetric multifocal brain lesions.
Elevation in serum aminotransferase levels.
Exclusion of similar diseases.[4]
Radiological findings are very helpful for differentiating the other types of post-infectious encephalopathies, such as Reye syndrome or acute disseminated encephalomyelitis.[5] Yamamoto et al., developed the ANEC severity score (ANEC-SS), which assigns risk based on the presence of circulatory shock, brainstem lesions, age over 48 months, thrombocytopenia below 100,000/µL, CSF protein above 60 mg/dL, all of which barring thrombocytopenia were associated with more severe sequelae.[6] Our patient had ANEC-SS 8/9. Despite the high severity score for ANEC, this child made a near full recovery, presumably because of early steroids, tocilizumab, IVIG and mito-cocktail.
Brain injury is presumed to result from a cytokine storm, and increased blood IL-6 levels in the acute phase of neurological illness correlate strongly with poor outcomes.[7] Figure 6 shows the mechanism of action of the prognosis of ANEC is poor: 30% mortality rate and <10% of survivors recovering fully.
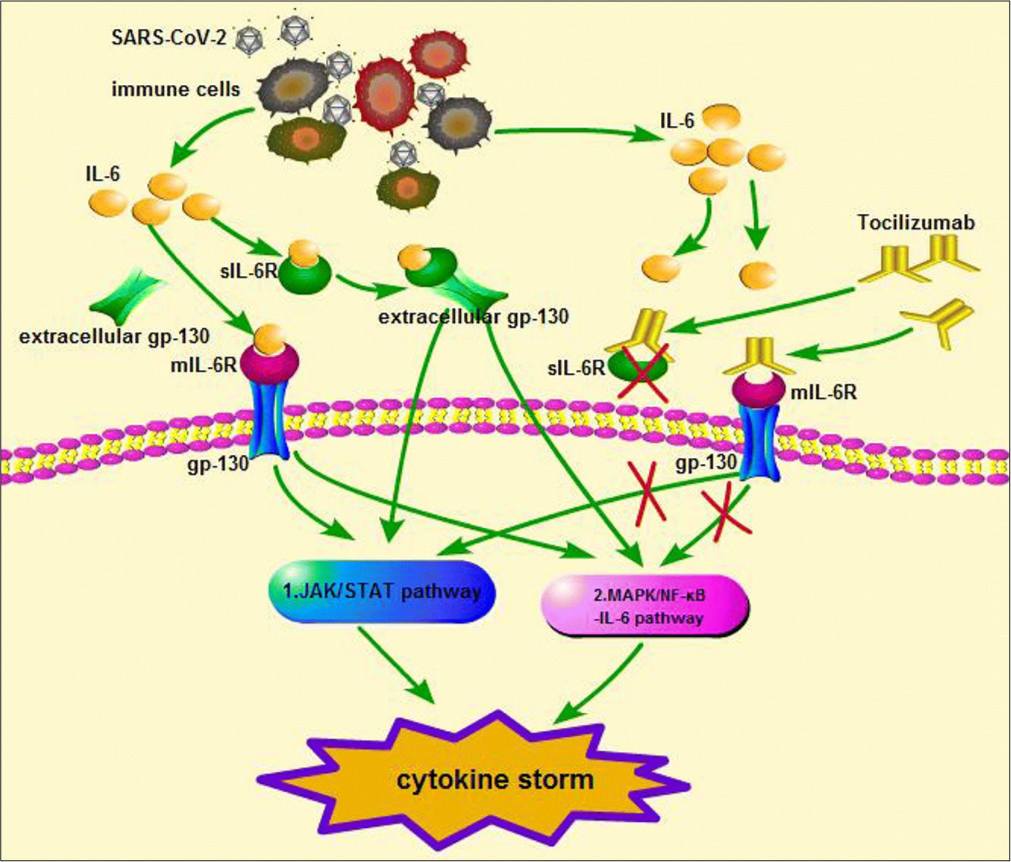
- Interleukin-6 (IL-6) triggers dimerisation of gp130 through binding of IL-6R. Tocilizumab binds to the IL-6R or the soluble IL-6R, which mediates IL-6 trans signalling, preventing binding of IL-6, hence reduces Janus kinase/signal transducer and activator of transcription signalling.
In ANEC, the current proposed treatment includes early steroid therapy, IVIG and/or plasma exchange to dampen the effects of hypercytokinaemia and hyperpermeability of the blood-brain barrier. However, neurological outcomes remain poor in severe ANEC. Second-line treatment options include therapeutic hypothermia and IL-6 blocking agents (tocilizumab). The use of cytokine inhibitors including tocilizumab and anakinra, in these conditions has been described as beneficial, although limited to small case series/ reports.[8] The current case reports on ANEC treatment suggest that early treatment of ANEC with tocilizumab (weight <30 kg: 12 mg/kg; weight ≥30 kg: 8 mg/kg) is safe and effective and has an important role in improving prognosis and preventing disability.[7] (Figure 6 shows the mechanism of action of tocilizumab[9]) In our case, the child received early steroid therapy, IVIG and tocilizumab. The second dose of tocilizumab was considered but withheld as there was a clinical improvement as well as a decrease in IL-6 levels.
Related studies also found that tocilizumab has the potential to exacerbate the occurrence of infections during treatment, such as pneumonia, and infections such as herpes zoster, which are the most common.[7,10] However, this child did not experience any adverse effects during treatment with tocilizumab.
CONCLUSION
This child, despite having high-risk ANEC with high ANEC-SS made a good recovery. We presume that early use of immunotherapy (tocilizumab in combination with MTP and IVIG), good supportive care and intensive neurorehabilitation has resulted in a good outcome for this child. The clinical presentation and management of this case will add to the pool of literature on the early use of tocilizumab in severe ANEC.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Case report: Acute necrotizing encephalopathy: A report of a favorable outcome and systematic meta-analysis of outcomes with different immunosuppressive therapies. Front Neurol. 2023;14:1239746.
- [CrossRef] [PubMed] [Google Scholar]
- Acute necrotising encephalopathy of childhood: A new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry. 1995;58:555-61.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical and imaging characteristics associated with neurological sequelae of pediatric patients with acute necrotizing encephalopathy. Front Pediatr. 2021;9:655074.
- [CrossRef] [PubMed] [Google Scholar]
- Acute necrotizing encephalopathy of childhood: A novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997;19:81-92.
- [CrossRef] [PubMed] [Google Scholar]
- Deep gray matter involvement in children with acute disseminated encephalomyelitis. AJNR Am J Neuroradiol. 1994;15:1275-83.
- [Google Scholar]
- A severity score for acute necrotizing encephalopathy. Brain Dev. 2015;37:322-7.
- [CrossRef] [PubMed] [Google Scholar]
- Favorable outcomes with early interleukin 6 receptor blockade in severe acute necrotizing encephalopathy of childhood. Pediatr Neurol. 2019;98:80-4.
- [CrossRef] [PubMed] [Google Scholar]
- SARS-CoV-2-related acute necrotizing encephalopathy of childhood with good response to tocilizumab in an adolescent. Pediatr Neurol. 2023;139:65-9.
- [CrossRef] [PubMed] [Google Scholar]
- Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig. 2020;40:511-8.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: A systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford). 2011;50:552-62.
- [CrossRef] [PubMed] [Google Scholar]






