Translate this page into:
Neonatal Group B streptococcal osteomyelitis and suppurative arthritis: A case report
*Corresponding author: Veerappan Somu, Department of Pediatrics, Kovai Medical Center and Hospital, Coimbatore, Tamil Nadu, India. veerasomu@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Duraiswamy A, Somu V. Neonatal Group B streptococcal osteomyelitis and suppurative arthritis: A case report. Karnataka Paediatr J 2020;35(2):117-20.
Abstract
Neonatal sepsis contributes significantly to neonatal morbidity and mortality. Group B streptococcus (GBS) is not a frequent cause of neonatal sepsis in India. Late onset sepsis by GBS presenting as focal infection like osteomyelitis is seen in only 3% of the total GBS sepsis profile in neonates. Here, we report a rare case of neonatal osteomyelitis with septic arthritis caused by GBS at an unusual site, the clavicle and sternoclavicular joint.
Keywords
Neonatal sepsis
Osteomyelitis
Septic arthritis
Group B streptococcus
INTRODUCTION
Neonatal sepsis continues to be a major global public health challenge.[1] Neonatal sepsis can be classified into early-onset sepsis and late-onset sepsis depending on the age of onset of symptoms. Neonatal sepsis is a clinical syndrome characterized by signs and symptoms of infection with or without bacteremia. It encompass various systemic infections such as septicemia, pneumonia, meningitis, osteomyelitis, arthritis, and urinary tract infections.[2] Gram-negative organism is the most common cause for neonatal sepsis with Klebsiella species being the most common organism as per the data available from India.[3-5]
Group B streptococcus (GBS) is an encapsulated gram positive diplococcus. Maternal colonization in the genital tract is the primary risk factor for GBS infection. Late onset sepsis by GBS manifests as bacteremia without a focus (65%), meningitis (25%), and cellulitis and osteoarthritis (2–3%) each.[6] The incidence of GBS infection was only 0.17 per 1000 live birth over a period of 10 years in a large tertiary care center in South India,[7] highlighting the rarity of GBS as an important neonatal pathogen. Low rate of maternal colonization and high prevalence of protective maternal antibody could be the reason for the low incidence of GBS sepsis. Existing data suggest that at least half of the infants with late onset disease by GBS are born preterm (before 37 weeks). Late onset disease has a lower fatality rate (1%–6 %) than early onset disease.[6]
Osteomyelitis in neonates is not so common. The global incidence of neonatal osteomyelitis is only 1–7 per 1000 NICU admissions.[8,9] Acute osteomyelitis is most commonly caused by Staphylococcus aureus and, less often, by GBS species and Gram-negative bacteria such as Escherichia coli and Klebsiella pneumonia.[10-12] Osteomyelitis due to GBS is common in humerus (56%), femur (24%), tibia and talus ( 4%), and others (ilium, clavicle, skull, digit, vertebrae, and ribs).[6] Clavicle is the first bone to ossify in human embryo and is the only long bone to ossify intramembranously. Literature review showed 16 cases of acute clavicle osteomyelitis in children and adolescents (age ranging from 0 to 16 years). Among these 16 cases, S. aureus was the most common organism isolated.[13-17]Septic arthritis is the invasion of a joint by an infectious agent resulting in joint inflammation. Global incidence of neonatal septic arthritis is approximately 0.3 per 1000 live births, whereas in India it has been reported as 0.6 per 1000 live births.[18] A study conducted in a tertiary care hospital in West Bengal showed, K. pneumoniae was isolated in more cases of neonatal septic arthritis, followed by S. aureus and E. coli.[19] Septic arthritis caused by GBS is most commonly encountered in hip (56%), knee (38%), and ankle joint (6%), respectively.[6]
CASE REPORT
A 14 days old Term/AGA/Female neonate, born by normal vaginal delivery with no significant antenatal, natal and immediate postnatal history was admitted with complaints of swelling over the right side of the chest [Figure 1]. There was no history of fever. On examination, baby was afebrile, icteric, and hemodynamically stable with a swelling over the medial end of right clavicle. Baby was moving right upper limb, with no cry on manipulation at right shoulder joint. The initial differentials considered were fracture clavicle/ abscess/osteomyelitis/Caffey’s disease/congenital syphilis.
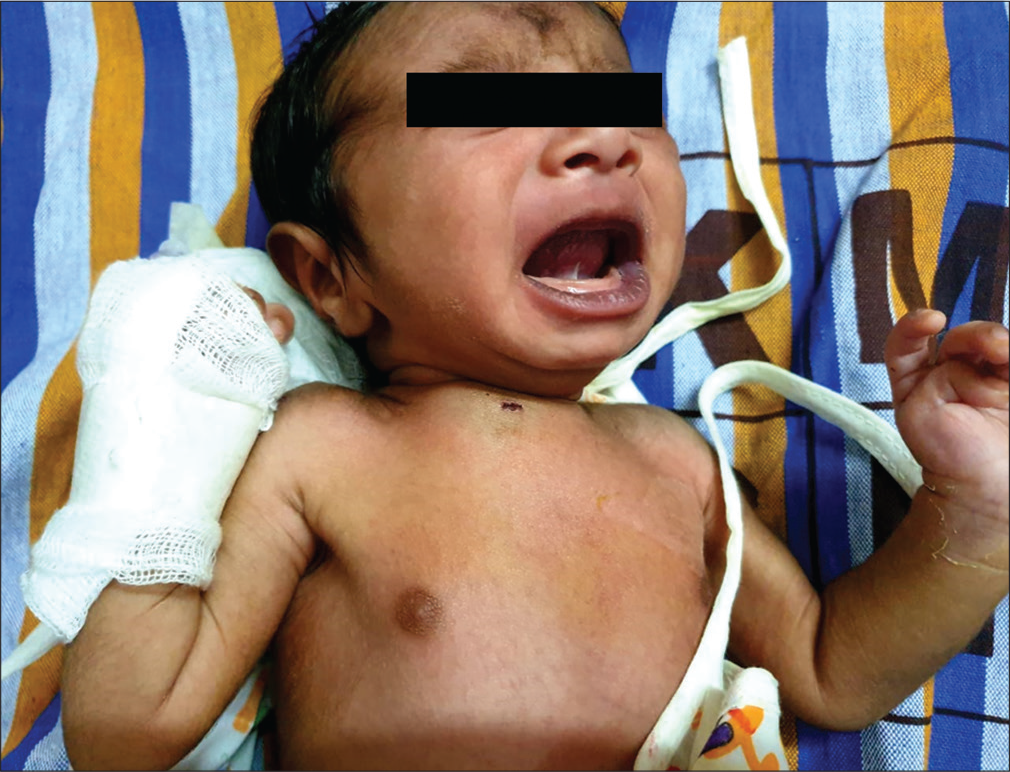
- Clinical picture.
Investigations revealed a blood leukocyte count of 35,200/mm3 with 58% neutrophils, 40% lymphocytes, and a platelet count of 799,000/mm3. CRP was 380 mg/dl. USG chest was suggestive of evolving abscess/hematoma [Figure 2]. On needle aspiration of the swelling around 1 ml of thick pus was recovered which showed many pus cells on Gram stain and subsequently showed growth of Group B beta-hemolytic streptococci (Streptococcus agalactiae). CT right sternoclavicular joint confirmed the diagnosis of right sternoclavicular septic arthritis with osteomyelitis of medial end of right clavicle [Figure 3]. Right sternoclavicular joint arthrotomy and osteomyelitis decompression of right clavicle were done. Child was started with Inj Ceftriaxone and oral linezolid. Child was given Inj ceftriaxone for 12 days and linezolid was continued for a total duration of 6 weeks. Child responded to treatment well and was on regular follow-up. Serial counts of CRP showed a declining trend. If resistant, we plan to workup for primary immuno-deficiency especially phagocytic defects.
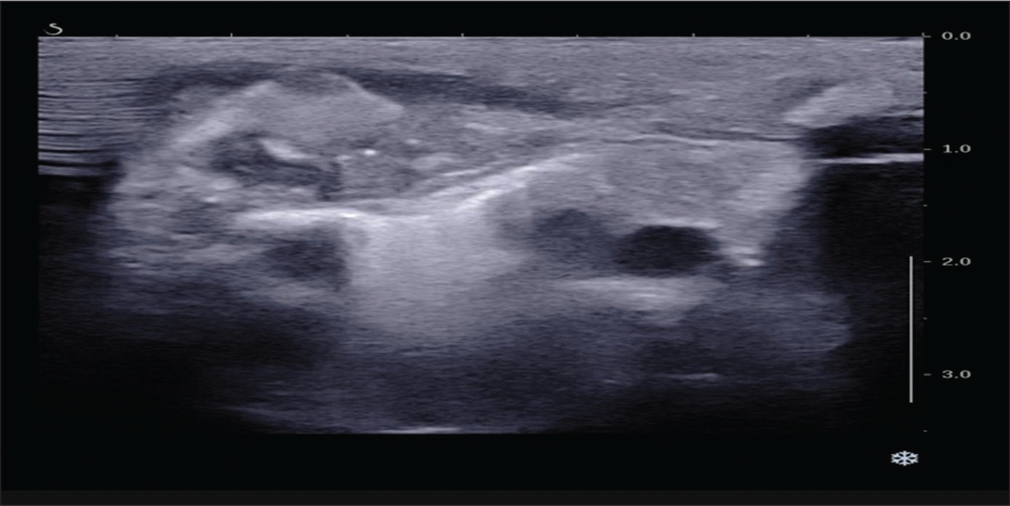
- USG chest
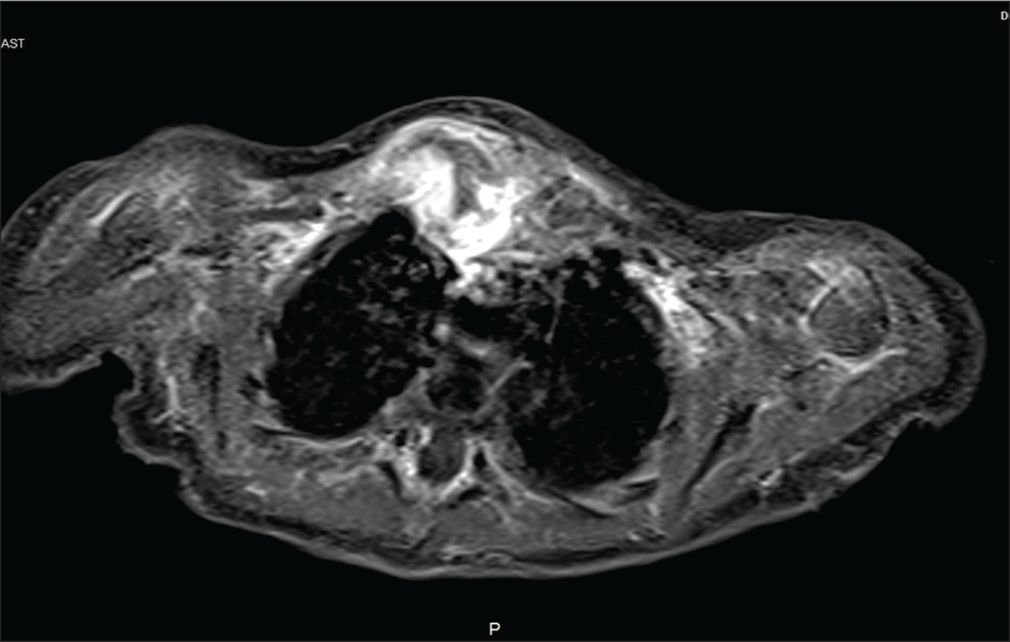
- CT sternoclavicular joint
DISCUSSION
Neonatal osteomyelitis occurs secondary to bacteremia and often insidious in onset. The proliferative vascular blood supply in the developing skeletal system of the neonates makes them prone to develop osteomyelitis. Metaphyseal region of the long bones especially the femur and the tibia is the most common sites involved. Osteomyelitis can also occur secondary to an extension from focal infection or bone trauma.[20] Other risk factors for osteomyelitis in preterm babies include umbilical catheterization and catheterization of other sites, especially the groin vessels. Septic emboli may form on vascular catheters, setting up a relatively high-inoculum bacteremia. Such risk can be reduced by following strict aseptic precautions while placing the lines. Studies have shown that urinary tract infection, periumbilical skin infections, and venous cut-downs for intravenous access also as risk factors for osteomyelitis.[21]
The long bones especially femur and tibia accounts for almost 50% cases of neonatal osteomyelitis, with humerus and the fibula being the next most commonly infected long bones.[21] Once bacterial infection sets in the metaphyseal vessels, inflammatory pathways begin leading to abscess formation. Abscess can rupture through the outside of the bone, forming a periosteal abscess, or through the side of the bone into the joint space, causing septic arthritis.[21]
Although Gram-negative bacilli account for 33% of neonatal sepsis, they only cause about 5% of cases of osteomyelitis. S. aureus being the most common organism causing neonatal osteomyelitis,[10] the incidence of methicillin-resistant S. aureus is increasing. GBS and coagulase-negative staphylococci rarely cause osteomyelitis. In preterm neonates Gram-negative organisms such as E. coli, K. pneumoniae, Enterobacter cloacae, Salmonella enteritidis, and Citrobacter freundii can cause osteomyelitis.[21] Although osteomyelitis from fungal infection is rare, the incidence is increasing.[21] Even in babies with disseminated congenital tuberculosis infection, osteomyelitis is rare.
Plain film radiography typically shows swelling of contiguous soft tissues; osteolytic and periosteal lesions, such as bone destruction and periosteal new bone elevations; and pathologic fractures, and typically of the long bones. Ultrasonography may not be specific for neonatal osteomyelitis.[22] Radionuclide bone scans are more sensitive than plain radiography in the early phase of the disease when X-ray changes have not appeared but are not always reliable. Conventional radiography still remains the first imaging modality because it is cheap and easily available. Periosteal and lytic changes are seen usually 10–21 days after onset of symptoms.[23] MRI provides more accurate details regarding the extent of the disease. Aspiration of the affected joint provide diagnostic clues and sometimes for therapeutic purposes.
The total duration of antibiotics is usually 4–6 weeks preferably by intravenous route.[21] To prevent recurrence
Appropriate selection of antibiotics and surgical drainage of pus if any plays an important role in treatment of osteomyelitis. Normally, anti-staphylococcal antibiotics such as nafcillin, cefazolin, or vancomycin along with a second-or third-generation cephalosporin (e.g., cefuroxime, and appropriate intravenous antibiotics should be given for at least 4 weeks, after resolution of the infection has been documented (e.g., negative culture results).[24] Jagodzinski et al. suggest a shortened course of treatment, in which patients with osteomyelitis are converted to oral antibiotics once they improve clinically and blood parameters improve.[25] Adequate pain control is another important aspect in the management of osteomyelitis especially in the early stages. cefotaxime) or an aminoglycoside (e.g., gentamicin, and tobramycin).[25] Antibiotic selection must be personalized to individual clinical factors and culture results. If there is no response to antibiotics, then surgical correction with necrotic debridement should be considered.
Even with early diagnosis and proper treatment, orthopedic sequelae of neonatal osteomyelitis are common which include cartilaginous growth plate destruction with discrepant limb length, angular deformity, pathologic fractures, arthritis, femoral condyle erosion, and limb palsy.[10] These changes can progress so a delayed diagnosis or inadequate treatment increases the risk for sequelae. Thus, all neonates with osteomyelitis should have careful outpatient follow-up for early detection of long-term orthopedic sequelae.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Neonatal sepsis: A major global public health challenge. Pediatr Infect Dis J. 2009;28:S1-2.
- [CrossRef] [PubMed] [Google Scholar]
- Microbiological profile of neonatal septicemia in a pediatric care hospital in Delhi. J Commun Dis. 2005;37:227-32.
- [Google Scholar]
- Clinical and bacteriological profile of neonatal infections in metropolitan city based medical college nursery. J Indian Med Assoc. 1999;97:3-5.
- [Google Scholar]
- Infectious Disease of the Fetus and Newborn Infants (7th ed). United States: Saunders; 2011.
- [Google Scholar]
- Neonatal group B streptococcal bacteremia in India: Ten years-experience. Acta Paediatr. 1999;88:1031-2.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome after acute osteomyelitis in preterm infants. Arch Dis Child. 1990;65:1060-2.
- [CrossRef] [PubMed] [Google Scholar]
- Unusual sites of acute osteomyelitis in childhood. Clin Radiol. 1982;33:222-30.
- [CrossRef] [Google Scholar]
- Acute staphylococcal osteomyelitis of the clavicle. J Pediatr Orthop. 1997;17:467-9.
- [CrossRef] [Google Scholar]
- Osteomyelitis of the clavicle: Clinical, radiologic, and bacteriologic findings in ten patients. Skeletal Radiol. 1994;23:205-10.
- [CrossRef] [PubMed] [Google Scholar]
- Nontraumatic clavicle lesions in children. J Pediatr Orthop. 1987;7:575-8.
- [CrossRef] [PubMed] [Google Scholar]
- Hematogenous osteomyelitis of the clavicle in children. Clin Orthop Relat Res. 1977;125:24-8.
- [CrossRef] [Google Scholar]
- Bone and joint infection in neonates. Indian J Pediatr. 1998;465:461-4.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal septic arthritis: Indian perspective. Eur J Rheumatol. 2020;7(Suppl 1):S72-7.
- [CrossRef] [Google Scholar]
- Methicillin-resistant Staphylococcus aureus osteomyelitis and septic arthritis in neonates: Diagnosis and management. Jpn J Infect Dis. 2007;60:129-31.
- [Google Scholar]
- Bone and joint infections in children. Pediatr Clin North Am. 2005;52:779-94.
- [CrossRef] [PubMed] [Google Scholar]
- Gram-negative neonatal osteomyelitis: Two case reports. Neonatal Netw. 2011;30:81-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective evaluation of a shortened regimen of treatment for acute osteomyelitis and septic arthritis in children. J Pediatr Orthop. 2009;29:518-25.
- [CrossRef] [PubMed] [Google Scholar]






