Translate this page into:
Prostaglandin E1-induced urticaria with harlequin change in a neonate with transposition of great vessels: A case report
*Corresponding author: H. A. Venkatesh, Department of Neonatology, Manipal Hospital, Bengaluru, Karnataka, India. venkatveena46@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Panigrahi NK, Venkatesh H. Prostaglandin E1-induced urticaria with harlequin change in a neonate with transposition of great vessels: A case report. Karnataka Paediatr J. 2024;39:88-90. DOI: 10.25259/KPJ_62_2023
Abstract
Prostaglandin E1 (PGE1) is an emergency drug used in neonates with critical congenital heart diseases (CHDs) to maintain the ductal patency. There are many adverse effects of this drug explained – cutaneous side effects being one of them, of which limited literature is available. In this case report, we describe a term neonate with critical CHD, started on PGE1 infusion to maintain ductal patency. Baby developed erythematous migratory annular rashes to harlequin colour change in a dose-dependent manner. The severity of rashes decreased, and there was a complete resolution with tapering down of drug doses to a minimum level to maintain a ductal patency. No antihistamines or steroids were administered for the treatment. The PGE1 should be tapered to a minimum dose to maintain the required saturation in case of severe cutaneous reaction and should not be stopped abruptly as it is a lifesaving drug in critical CHD.
Keywords
Neonate
Cyanotic congenital heart disease
Prostaglandin E
Urticaria
Harlequin change
INTRODUCTION
Prostaglandin E1 (PGE1) is an emergency drug used in neonates with critical congenital heart diseases (CHDs) to maintain the ductal patency.[1] There are many adverse effects of this drug explained in the literature. The most common side effect is apnoea. The limited literature is available describing the adverse cutaneous manifestations of prostaglandin E1 infusion. We present a neonate on PGE1 developing an extensive erythematous rash with a harlequin color change.
CASE REPORT
An early-term male neonate was born to a non-consanguineous couple by lower-section cesarean section. He required intubation at birth, requiring high oxygen therapy to maintain saturation of 50–60%. The echocardiography demonstrated the transposition of great vessels with shunts, atrial septal defect and patent ductus arteriosus. He was started on PGE1 infusion @ 100 ng/kg/min. Atrial septostomy was performed on 2nd day of life. He developed an erythematous blanchable rash on day 3 of life involving the scalp, the face, and the upper trunk with sharp demarcation between the upper and lower abdomen, suggesting harlequin [Image 1]. The rash gradually progressed to erythematous annular migratory blanchable rash by day 4 of life when prostaglandin was gradually tapered to 60 mcg/kg/min [Image 2]. He was also started on antibiotics for suspected sepsis. Gradually, the prostaglandin was weaned to 20 ng/kg/min; the cutaneous lesions also disappeared [Image 3]. He continued to be stable hemodynamically on the ventilator, and his septic markers were normal. On day 7 of life, he was taken for corrective surgery for transposition of the great arteries and discharged home on direct breastfeeding.
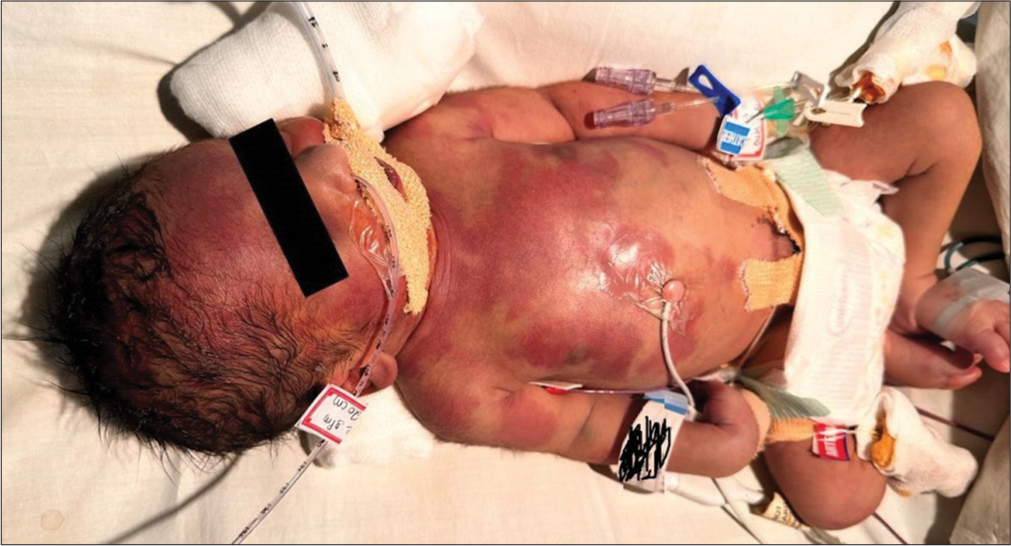
- Diffuse erythematous rash at prostaglandin E1 of 100 ng/kg/min.
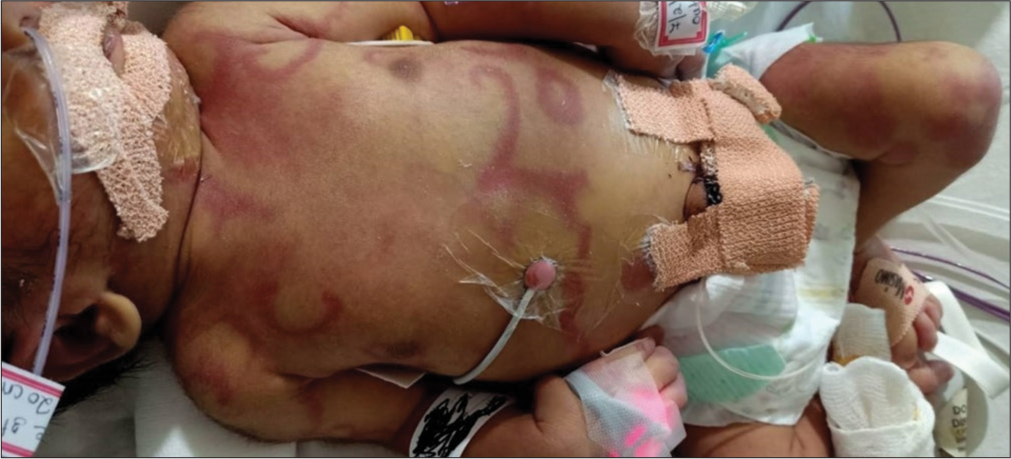
- Annular migratory rash at prostaglandin E1 of 40 ng/kg/min.
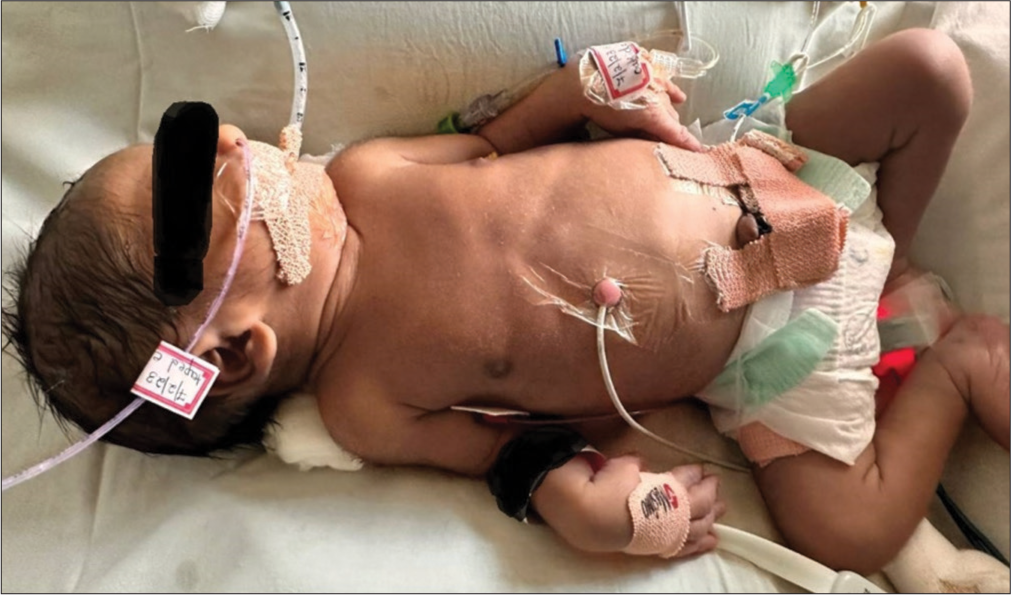
- Complete resolution of rash at prostaglandin E1 of 20 ng/kg/min.
Management and outcome
In view of the erythematous rashes, the prostaglandin dose was tapered to a minimum therapeutic dose to maintain the target saturation. At the dose of 20 ng/kg/min, the rashes completely disappeared, and there was no further recurrence. No antihistamines or steroids were administered in our case for the skin lesions.
DISCUSSION
PGE1-related dermatological complications are not much reported in the neonatal population. After an extensive search, we could find only six cases characterized by migratory polycyclic erythema to harlequin changes.[2-4] To the best of our knowledge, this was the first case being reported from India. Similar case reports were published by Young et al. and Carter and Garzon.[2-4] Both the case reports described dose-dependent cutaneous reactions as seen in our index case.
PGE1 acts by activating adenylate cyclase and adenosine triphosphate sensitive potassium channel and, causing vasodilation and keeping the ductus arteriosus patent. The drug can induce vasodilatation and cause cutaneous inflammation through a non-allergic pathway of histamine release. Hypotension, rhythm disturbance, seizures, congestive cardiac failure, hypo- or hyperkalaemia, cortical proliferation, and hyperostosis of long bones[5] are the other adverse side effects of the drug, with apnoea being the most common seen in clinical practice. The harlequin color change generally resolves spontaneously without any residual skin reaction.[6,7] The non-allergic mechanism of histamine release causes the urticarial rash, and the severity is dose dependent. Similar skin reaction is noted secondary to vancomycin causing red man syndrome[8] and severity being dependent on dose and rate of infusion. Histopathological examination demonstrated sparse mononuclear cells. None of the medications, including the antihistamines and steroids, neither local nor systemic, will help in the management of such rash. The PGE1 should be tapered to a minimum dose to maintain the required saturation and should not be stopped abruptly as it is a lifesaving drug in critical CHD. The prompt recognition of cutaneous side effects of prostaglandin allows for suitable diagnosis and avoidance of unnecessary interventions and treatments, including antihistamines and topical or systemic steroids. As no evidence of systemic sequelae has been reported till now, the continuation of the lifesaving PGE1 treatment is warranted for critical CHD until the palliative or definitive surgery is performed.
Lessons learnt
Prostaglandin-related cutaneous effects present in varied morphology in a dose-dependent manner
Minimum effective dose of PGE1 to be continued to maintain ductal patency in critical CHD
Prompt recognition and diagnosis avoids unnecessary interventions.
CONCLUSION
Prostaglandin induced urticaria can be polymorphic in presentation, hence prompt diagnosis and cautious management is necessary in a neonate where it is a life saving drug.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Administration of prostaglandin E1 in neonates with critical congenital cardiac defects. J Pediatr. 1978;93:481-5.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal urticaria due to prostaglandin E1. Pediatr Dermatol. 2000;17:58-61.
- [CrossRef] [PubMed] [Google Scholar]
- The harlequin color change and association with prostaglandin E1. Pediatr Dermatol. 2004;21:573-6.
- [CrossRef] [PubMed] [Google Scholar]
- An unusual migratory polycyclic eruption after administration of prostaglandin E in a neonate. JAAD Case Rep. 2016;2:377-9.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal Harlequin color change associated with Prostaglandin E1 administration. Pediatr Int. 2021;63:610-1.
- [CrossRef] [PubMed] [Google Scholar]
- Harlequin color change in an infant during anesthesia. Anesthesiology. 1985;62:695.
- [CrossRef] [PubMed] [Google Scholar]
- Picture of the month. Harlequin color change. Arch Pediatr Adolesc Med. 1995;149:1171-2.
- [CrossRef] [PubMed] [Google Scholar]
- Vancomycin and the red-man syndrome: Pharmacodynamics of histamine release. J Infect Dis. 1988;157:502-7.
- [CrossRef] [PubMed] [Google Scholar]






