Translate this page into:
Recent advances in cerebral palsy
*Corresponding author: Vykuntaraju K. Gowda, Department of Pediatric Neurology, Indira Gandhi Institute of Child Health, Bengaluru - 560 027, Karnataka, India. drknvraju08@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gowda VK. Recent advances in cerebral palsy. Karnataka Paediatr J 2020;35(1):4-18.
Abstract
The words unpreventable, incurable, and untreatable are still synonymous with cerebral palsy (CP). However, research and evidence coming from the fields of neuroplasticity, neuroregeneration, and neuroprotection provide considerable cause for optimism for children with CP. There are now at least 64 different interventions for CP seeking 131 outcomes. A search of the Cochrane Library, PubMed, and Google Scholar was made using the keywords: CP, static encephalopathy, birth asphyxia, perinatal insult, hypoxic-ischemic encephalopathy, and neonatal encephalopathy. We found evidence to suggest that following interventions: Anticonvulsant drugs, ankle casting, botulinum toxin for focal spasticity, bisphosphonates, diazepam, hip surveillance, and dorsal rhizotomy are effective. The following interventions improve function: Bimanual training, constraint-induced movement therapy, context focused therapy, goal-directed/functional training, home programs, and occupational therapy. These interventions are effective if started early in life. Therapies such as hyperbaric oxygen, hip bracing, and neurodevelopmental therapy when child contractures are already developed are ineffective. In the last decade, the evidence on CP has rapidly expanded, providing clinicians and families with the possibility of newer, safer, and more effective interventions. In this update, the author reviews the current evidence of the management of CP and provides a comprehensive evaluation and multidisciplinary management.
Keywords
Cerebral palsy
Birth asphyxia
Hypoxic-ischemic encephalopathy
Early intervention
Multidisciplinary management
Early intervention requires early identification
Of infants with possible cerebral palsy
INTRODUCTION
Cerebral palsy (CP) is a well-recognized neurodevelopmental condition beginning in early childhood and persisting through the lifespan. Originally reported by Little in 1861 and originally called “cerebral paresis.”[1] The incidence of CP is 2–2.5/1000 live births[2] and the resulting disability varies from mild to total dependence. “The definition of CP describes a group of disorders of the development of movement and posture, causing activity limitation that is attributed to non-progressive disturbances that occurred in the developing fetal or infant brain. The motor disorders of CP are often accompanied by disturbances of sensation, cognition, communication, perception, and/or behavior, and/or by a seizure disorder.”
ETIOLOGY OF CP
W. J. Little in the 1840s, assertion that nearly all cases of CP what he called spastic rigidity of newborns resulted from preterm birth or asphyxia at birth has left an enduring mark on subsequent thinking about the etiology. Later Sigmund Freud cautioned against assuming these two factors as fully causal, but only in the latter half of the 20th century did research begin to illustrate the complex nature of this disease and associated etiological factors. Present-day evidence suggests that about 80% of CP is caused by an in-utero brain injury; only 10% occurs around the time of birth and 10% occurs in early childhood.[3] In a recent systematic review, ten risk factors have been reported to be significantly associated with CP and these are placental abnormalities, major and minor birth defects, low birth weight, meconium aspiration, emergency cesarean section, birth asphyxia, neonatal seizures, respiratory distress syndrome, hypoglycemia, and neonatal infections.[4] Various other risk factors are shown in [Table 1]. In India, still, perinatal risk factors are a major cause of CP. A study done by Gowda et al. showed that birth asphyxia is the main risk factor in 45% of children with CP.[5]
| Pre-natal (Maternal/fetal/placental) | Perinatal | Post-natal |
|---|---|---|
| Iodine deficiency, iron deficiency, and poor nutrition Intrauterine infections (TORCH), high fever, UTI Chorioamnionitis Hypertension Maternal diseases, for example, diabetes, hypertension, hyperthyroidism Teratogens – drugs, radiation, smoking, alcohol, and environmental toxins Fertility problems, for example, advanced age at conception, history of infertility, recurrent fetal wastage Poor antenatal care Poor socioeconomic status |
1.Birth asphyxia 2.Prematurity 3.Intrauterine growth retardation 4.Hyperbilirubinemia 5.Intraventricular and intracerebral bleeds 6.Hypoglycemia, dyselectrolytemias 7.Sepsis, pneumonia, and meningitis 8.Premature separation of placenta |
Neuroinfections Viral encephalitis Tubercular meningitis Pyogenic meningitis Head injuries Anoxia Suffocation Electrocution Post-operative cardiac arrest Post-epileptic Cerebrovascular accidents/strokes Gastroenteritis and dehydration |
NEUROPATHOLOGICAL ASPECTS OF PERINATALLY ACQUIRED CP IN PRETERM AND FULL TERM
For better conceptualization of topic, pathology can be divided into three parts: Encephalopathy of prematurity, ischemic injury in term infant, and perinatal stroke. Major neuropathological varieties of neonatal hypoxic-ischemic encephalopathy are selective neuronal necrosis, parasagittal cerebral injury, periventricular leukomalacia (PVL), and focal (and multifocal) ischemic brain necrosis-stroke
SELECTIVE NEURONAL NECROSIS: PATTERNS OF INJURY
Selective neuronal necrosis is the most common variety of injuries observed in neonatal hypoxic-ischemic encephalopathy. Three basic patterns derived from clinical and brain imaging are diffuse- very severe and very prolonged, cortex with deep nuclear (putamen, thalamus) – moderate to severe and prolonged and deep nuclear with brain stem-severe and abrupt. Two other patterns, pontosubicular neuronal injury and cerebellar injury, occur, particularly in premature infants. The development of CP can be considered the result of a remarkable series of events that occur in the brain during its development. Understanding etiological factors and pathways involved in its pathogenesis is utmost importance for treatment and exploring newer therapeutic options. [Table 2] shows the clinicopathological correlation and neurological outcome of CP.
| CP subtype | Pathology | Underlying etiology | Neurological outcome |
|---|---|---|---|
| Spastic diplegia | Periventricular leukomalacia | Prematurity | Visual impairment, hyperactivity |
| Spastic quadriplegia | Multicystic encephalopathy cerebral malformation | Perinatal/intrauterine hypoxic-ischemic events | Decreased IQ seizures bulbar weakness |
| Spastic hemiplegia | Cerebral injury (infarction, necrosis) | Pre-natal events like hypoperfusion, hemorrhage, Genetic | Seizures, learning problems |
| Dyskinetic | Basal ganglion – status marmoratus due to bilirubin deposition | Perinatal asphyxia bilirubin-induced neurological dysfunction – BIND (kernicterus) | Hearing impairment |
| Ataxic | Cerebellar lesions | Pre-natal (genetic) | Motor delay |
BIND: Bilirubin-induced neurologic dysfunction
TYPES AND CLASSIFICATIONS OF CP
It is understandable that in such a diverse collection of disorders, many attempts at classification should be of limited value. [Table 3] shows the various classification of CP.
| Physiologic | Topographic | Functional – GMFCS/walking | Etiological |
|---|---|---|---|
| Spastic Dyskinetic 1. Dystonic 2. Choreoathetotic Ataxic Mixed |
Diplegia Hemiplegia Quadriplegia Monoplegia Triplegia Double hemiplegia |
Class I: Walks without limitations Class II: Walks with limitations Class III: Walks using a hand-held mobility device Class IV: Self-mobility with limitations; may use powered mobility Grade V – Transported in a manual wheelchair |
Pre-natal natal post-natal |
GMFCS: Gross motor function classification system
Classification of CP subtypes based on Surveillance for CP in Europe (SCPE) shown in [Figure 1]. Adapted from SCPE plenary meeting, held in Oxford, 1999.[6]
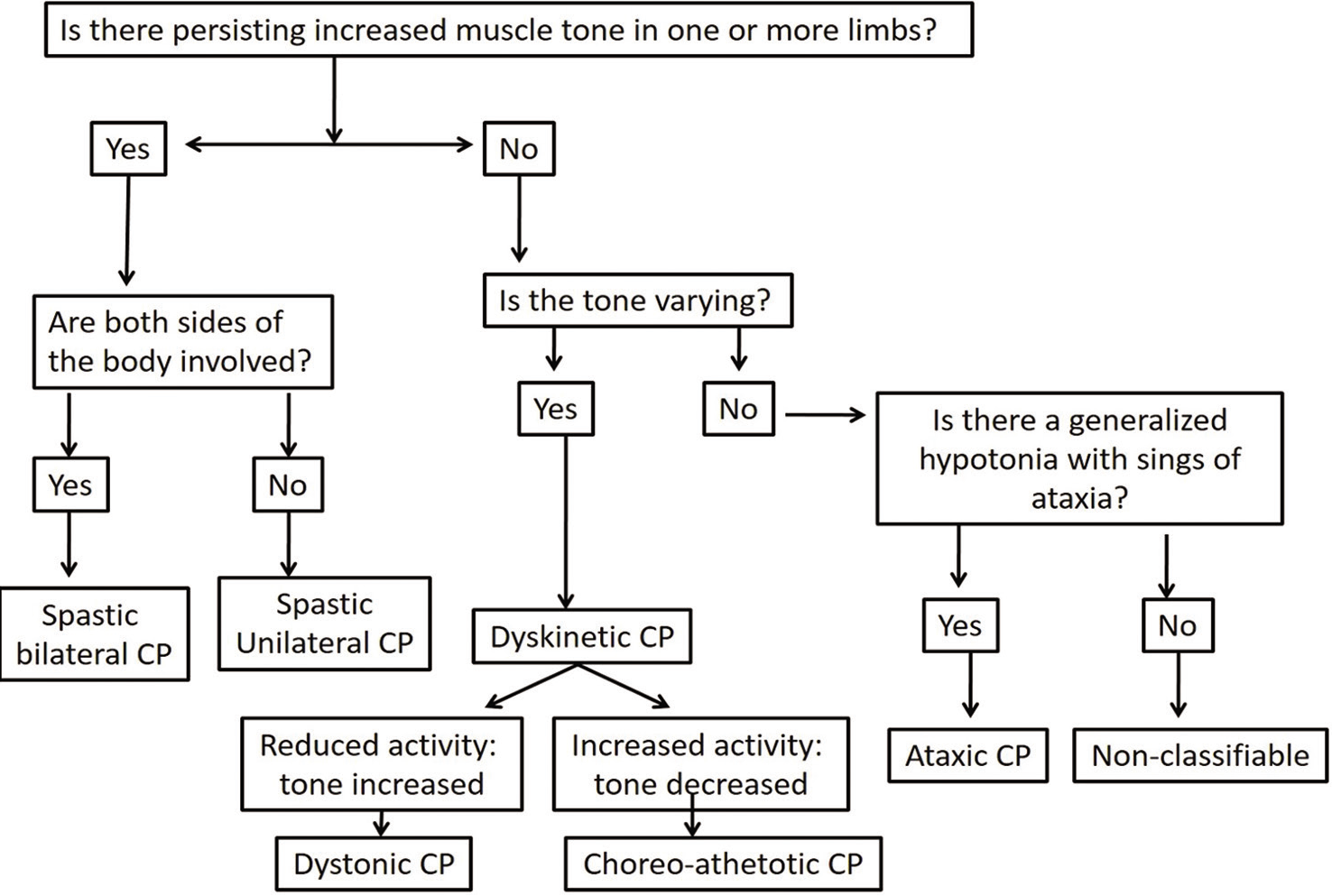
- Flow diagram showing Surveillance for Cerebral Palsy in Europe (SCPE) classification of cerebral palsy. Hierarchical classification tree of sub-types (adapted from DMCN2000).
The classification of subtypes of CP is based on clinical features and predominant neurological findings. It identifies three main groups: Spastic, dyskinetic, and ataxic CP. All subtypes of CP have an abnormal pattern of movement and posture. Additional features include:
-
Spastic CP
Unilateral spastic CP – earlier called hemiplegic CP
Bilateral spastic CP – it can be a diplegic or quadriplegic type.
-
Dyskinetic CP
Dystonic CP is dominated by decreased movements with increased tone
-
Choreathetotic CP dominated by increased movements with decreased tone.
The same child can have both spasticity and dystonia in mixed CP. The dominating features should determine subtype classifications and can be labeled as mixed CP dyskinetic with spastic when dystonia more than spasticity vice versa.
Ataxic CP is characterized by – loss of orderly muscular coordination.
CP MIMICS
All children with features of CP should be carefully evaluated for an underlying cause, particularly in the presence of red flag features shown in [Table 4].[7,8] CP mimics can be grouped on the basis of age of presentation or clinical examination and history. They can be grouped into a subtype of CP, as shown in [Table 5].[7-9] Ataxic and dyskinetic syndromes are particularly liable to cause confusion. This important distinction between a progressive and non-progressive disorder is made on clinical grounds and appropriate investigations when indicated.
| History | Examination | Neuroimaging |
|---|---|---|
| Positive family history of similar disease | Dysmorphic facies | Normal MRI of brain |
| History of consanguinity | Neurocutaneous markers | Isolated abnormal signals from globus pallidus. |
| Absence of sentinel events | Isolated muscular hypotonia | Imaging features are not suggestive of cerebral palsy |
| No risk factors for CP | Paraparesis | Cerebellar atrophy |
| Neurodevelopmental stagnation or regression | Peripheral nervous system involvement (pes cavus) | Demyelination |
| Episodic decomposition | Optic atrophy/retinopathy | |
| Fluctuation in motor functions | Systemic signs |
MRI: Magnetic resonance imaging
| S. No. | Condition/disease |
|---|---|
| Conditions presenting with true muscle weakness | |
| 1 | Duchenne muscular dystrophy, hereditary motor sensory neuropathy, myopathies |
| 2 | Infantile neuroaxonal dystrophy – INAD |
| 3 | Mitochondrial cytopathies |
| 4 | Cerebral white matter diseases – hypomyelinating leukodystrophies |
| Conditions with significant dystonia or involuntary movements | |
| 1 | DOPA responsive dystonia |
| 2 | PKAN – pantothenate kinase-associated neurodegeneration |
| 3 | Pyruvate dehydrogenase deficiency, Leigh syndrome, and other mitochondrial disorders |
| 4 | Glutaric aciduria type I and other organic acidurias |
| 5 | Juvenile neuronal ceroid lipofuscinoses |
| 6 | Rett syndrome |
| 7 | Pelizaeus-Merzbacher disease |
| 8 | Lesch-Nyhan syndrome |
| Conditions with predominant spastic diplegia or quadriplegia | |
| 1 | Adrenoleukodystrophy – ALD |
| 2 | Arginase deficiency |
| 3 | Metachromatic leukodystrophy |
| 4 | Hereditary progressive spastic paraplegia |
| 5 | Holocarboxylase synthetase deficiency |
| 6 | Pre-natal iodine deficiency (“neurological cretinism”) |
| 7 | TORCH infections |
| Conditions with ataxia (ataxic CP is rare) | |
| 1 | Angelman syndrome |
| 2 | Niemann-Pick disease type C |
| 3 | Ataxia-telangiectasia |
| 4 | Pontocerebellar hypoplasia or atrophy |
| 5 | Chronic/adult GM2 gangliosidosis |
| 6 | Mitochondrial cytopathy (NARP mutation) |
| 7 | Posterior fossa tumors |
| 8 | Joubert’s syndrome |
| Conditions with significant bulbar and oral-motor dysfunction- Worster-drought syndrome/ perisylvian/ opercular syndrome | |
| 1 | Polymicrogyria |
| 2 | Zellweger syndrome |
EARLY PREDICTORS IN CP
Early diagnosis is very important for early intervention and thus determines the outcome. It also helps in counseling worried parents appropriately. Early markers of CP can be identified based on neurological examination and evolution of signs in CP, general movement assessment, and neuroimaging studies. The great advantage of detecting an increased risk of CP at such an early stage consists of the possibility of intervention long before the emergence of obvious pathological features of CP.
Some of the commonly used neurological examination tools in the high-risk clinic are the Hammersmith Infant Neurological Examination (HINE), the Amiel-Tison scale, The Bayley Scales of Infant and Toddler Development, and Dubowitz neonatal neurological examination.
HINE
It is a well-studied neurological exam in healthy or high- risk infants. The HNE is easy to perform. It is relatively brief and standardized. It is a scorable clinical neurological examination. It is an application to in the age group of 2 months–24 months. It is easily accessible to all clinicians. It has good inter-observer reliability, even in less experienced staff. It has no associated costs such as lengthy certifications or proprietary forms. The use of the HINE optimality score and cutoff scores provides prognostic information on the severity of the motor outcome. The HINE can further help to identify those infants needing specific rehabilitation programs. It includes 26 items assessing cranial nerve function, posture, quality and quantity of movements, muscle tone, and reflexes and reactions. Each item is scored individually such as 0, 1, 2, or 3. The sum score of all individual items ranges from 0 to 78. A questionnaire with instructions and diagrams is included on the scoring sheet, similar to the Dubowitz neonatal neurological examination. HINE score allows the identification of early signs of CP and other neuromotor disorders if apply sequentially. Individual items predict motor outcomes. For example, in preterm infants assessed between 6 and 15 months corrected age, scores above 64 predict independent walking with a walked sensitivity of 98% and specificity of 85%. Conversely, scores below 52 were highly predictive of CP and severe motor impairments.[10]
COMPREHENSIVE EVALUATION OF CHILDREN WITH CP
The comprehensive evaluation and care of a child with CP can be simplified into the following five steps: Confirming the diagnosis and determining the cause, assembling “the team,” assessing functional abilities, determining goals of care, and comprehensive care initiation
Step 1: Confirming the diagnosis and determining the cause
This step includes a detailed history taking and examination followed by necessary investigations such as computed tomography (CT) or magnetic resonance imaging (MRI) of brain and ancillary investigations such as electroencephalography (EEG), metabolic, genetic, and coagulopathy testing. American Academy of Neurology (AAN) recommendations on neuroimaging:[11] Neuroimaging is recommended in the evaluation of a child with CP if the etiology has not been established. MRI is better than CT scanning as the yield of MRI is higher and helps in the identification of timing of insult (Level A, Class I-III evidence).
Step 2: Assembling the team
This will include a coordinated approach between various branches of medicine in providing complete care to a child with CP.
Step 3: The complete assessment
This step includes a comprehensive and extensive evaluation of the functional abilities, comorbidities, and the support system of children with CP. It can be further subdivided into the following steps:
Mobility and motor impairment evaluation
Associative conditions assessment
Activities of daily living evaluation
Family dynamics and socioeconomic status assessment
-
Educational assessment.
Muscle tone: Modified Ashworth Scale is used for tone assessment, as shown in [Table 6].
Associative conditions assessment: AAN recommendation on additional testing for comorbidities:[11] Due to the high incidence of associated conditions, children with CP should be screened for “intellectual disability, ophthalmologic and hearing impairments, and speech and language disorders” (Level A, Class I and II evidence). Monitoring should be done for nutrition, growth, and swallowing dysfunction. If screening tests are suggestive of impairments, it should be confirmed by other diagnostic tests.
Activities of daily living evaluation: The following points are to be noted to give appropriate assistance as per the impairment, bathing, dressing and undressing, eating, food preparation, grooming, housekeeping, leisure, and play; recreation, personal hygiene, mobility, self-care, shopping, transferring (bed, chair, toiletry, etc.), and work
| Grade 0 | No increase in muscle tone |
|---|---|
| Grade 1 | Slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion when the affected part(s) is moved in flexion or extension |
| Grade 1+ | Slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM |
| Grade 3 | More marked increase in muscle tone through most of the ROM, but affected part(s) easily moved |
| Grade 3 | Considerable increase in muscle tone, passive movement difficult |
| Grade 4 | Affected part(s) rigid in flexion or extension |
Step 5: Coordinated, comprehensive care plan implementation
After a detailed evaluation, multidisciplinary care is implemented with the help of the team gathered so as to achieve the goals set. Ashwal et al.[11] have provided a practice parameter for diagnostic assessment and evaluation of a child with CP, which provides a comprehensive flow chart for evaluation.[11]
COMORBIDITIES IN CHILDREN WITH CP
CP is often accompanied by disturbances of sensation, perception, cognition, communication, behavior, epilepsy, and secondary musculoskeletal problems. This definition has led not only to an increase in awareness of the occurrence of comorbidities in individuals with CP but also the need for interdisciplinary management of these comorbidities to improve the life span and quality of life of children with CP. Brown et al. defined comorbidity as any disorder associated with CP, but which can also occur as a stand-alone disorder in individuals without CP.[12] Comorbidities occurring in Children with CP are shown in [Table 7]. They categorized types of comorbidities in individual with CP:
Comorbid/co-occurring: Disorders not caused by the injury to the developing brain, nor are complications of the main CP condition
Co-casual: Disorders caused by the same injury to the developing brain that caused CP (i.e., epilepsy and cognitive impairment)
Complications: Disorders that are complications of the main CP condition (i.e., scoliosis and hip dislocation)
| S. no. | Neurologic disorders | Medical disorder | Psychiatric disorders |
|---|---|---|---|
| 1. | Seizure/ Epilepsy | Nutrition and growth | ADHD |
| 2. | Intellectual disability | Gastrointestinal 1.Feeding problems 2.Dysphagia 3.GERD 4.Constipation |
Autism spectrum disorder |
| 3. | Speech | Respiratory 1.Obstructive sleep apnea 2. Parenchymal lung disease due to aspiration 3. Restrictive lung disease due to severe kyphoscoliosis 4.Insufficient coughing |
Behavioral disorders |
| 4. | Sleep disorders | Genitourinary 1.Urinary incontinence 2.Detrusor hyperactivity 3.Recurrent UTI 4.Detrusor sphincter dyssynergia |
Depression |
| 5. | Spasticity | Orthopaedic 1.Contracture 2.Subluxations 3.Bony deformities 4.Osteopenia |
Learning problems |
| 6. | Dystonia | Hearing impairment and visual impairment | Anxiety |
SEIZURES IN CP
Seizures are frequently encountered in children with CP. The frequency of seizures in children with CP is 40 times higher than the general population. Epilepsy in CP modifies the course of CP, it complicates rehabilitation, and it also influences motor and intellectual function. It can be life- threatening also. Various studies show, on an average, 43% (range 35–62%) of children with CP have epilepsy.
RISK FACTORS FOR EPILEPSY IN CP[13]
The presence of neonatal seizures
Low Apgar score (≤4 points)
Extremely preterm infants (≤31 weeks of gestation)
Neonatal resuscitation
Family history of epilepsy
CP caused by pre-natal factors, especially cerebral dysgenesis
Intrauterine infection (especially herpes encephalitis).
Hemiplegic and tetraplegic forms of CP
Severe intellectual disability
The presence of epileptiform discharges on the EEG.
CHARACTERISTICS OF EPILEPSY IN CP
Despite the wide polymorphism of clinical cases, epilepsy in combination with CP has a number of common characteristics. They can be expressed as the following features.
In the majority of cases (up 74.2%), epilepsy in children with CP occurs within the 1st year of life
Children with CP have a broad spectrum of epilepsies – varying from favorable combinations with benign forms to extremely severe epileptic encephalopathies (Ohtahara, West, Lennox-Gastaut syndromes, etc.)
Seizures often need polytherapy
There is increased risk of seizures going into status epilepticus
Increased risk of recurrence of epilepsy in children with CP after antiepileptic drugs (AED) are discontinued
Seizure free period of 1 year is achieved in children with normal intelligence, children on monotherapy, spastic diplegia subtypes, and children having single seizure type.
CP is the most common cause for West syndrome in India, the history of spasms should be asked as most of the time, epileptic spasms are missed and if not treated early, the long- term outcome of CP is poor.
CHALLENGES IN IDENTIFYING SEIZURES IN CP
Epileptic seizures may be difficult to distinguish from other involuntary movements, particularly in dystonic/ dyskinetic or ataxic CP
Children with CP may have breath-holding spells, reflex anoxic attacks, vasovagal syncope, and other types of non-epileptic paroxysmal disorders
Gastroesophageal-reflux disease (GERD-Sandifer syndrome) is commonly seen in CP
Consider seizures in the differential diagnosis of any unexplained worsening of the motor disorder in CP, sudden falls, a cognitive decline or a decrease in alertness
CP and intellectual disability: Unable to describe the epileptic events themselves, parents may not recognize subtle seizure manifestations.
Diagnostic delay is associated with a 7.4-point drop in Vineland Scales of Adaptive Behavior motor score, 8.4-point drop in processing speed on Wechsler Intelligence Scale for Children (WISC) and 14.5-point drop-in full-scale intellectual quotient (IQ) on WISC.
ROLE OF EEG
Consider when history or examination is suggestive of epilepsy or epilepsy syndrome. Not useful in predicting the development of seizures in a child with CP. When there is difficulty in differentiating seizures from dyskinetic movements and there is a history of doubtful myoclonic jerks, EEG has to be done. EEG is useful for diagnosis of seizure type, identification of epilepsy syndrome, prediction of long-term outcome, severity, and monitoring.
TREATMENT
The principles of drug therapy in children with CP and epilepsy are the same as those for children with epilepsy in general. The type of seizure, epilepsy syndrome, age, gender, cost, the side effect profile of the medicine being considered, interactions with other possible medications, and associated comorbidities guide the selection of AEDs. In general, the drugs of the first choice for focal seizures are oxcarbazepine and carbamazepine should be avoided in CP as they can aggravate myoclonic jerks. In the case of infantile spasms, injectable or oral steroids and vigabatrin should be considered.
NEUROIMAGING IN CP
Neuroimaging should be done in all cases of CP of unknown origin. Although the diagnosis of CP is clinical, neuroimaging helps in establishing etiology and timing of insult and identifying malformations which have genetic underpinnings.[11]
Identifying etiology is important especially for,
Genetic counseling (recurrence risk and pre-natal diagnosis in genetic etiology)
Avoids further unnecessary testing
Medicolegal cases.
Neuroimaging-which one to choose?
Cranial ultrasonography-perinatal period
CT scan of brain, yield is 77%. Poor for dyskinetic CP, good for hemiparetic CP. Picks up TORCH infection and identifies surgically treatable cause in ~5% of children like hydrocephalus
MRI of brain, yield is 89%. Helps in assessing the timing of insult such as pre-natal, perinatal, or post-natal. Good for prematurity associated CP/PVL. Better for dyskinetic CP to look for basal ganglia and thalamus and also malformation of brain.
Lesions and type of CP
PVL: Spastic diplegia
Basal ganglia-dyskinetic CP
Focal lesions – e.g., porencephalic cyst – spastic hemiplegia.
Multidisciplinary management of CP
CP rehabilitation is a complex process aiming at ensuring children and their families the best possible quality of life. A child with CP should be managed within an integrated multidisciplinary team with appropriate expertise [Figure 2].[14]
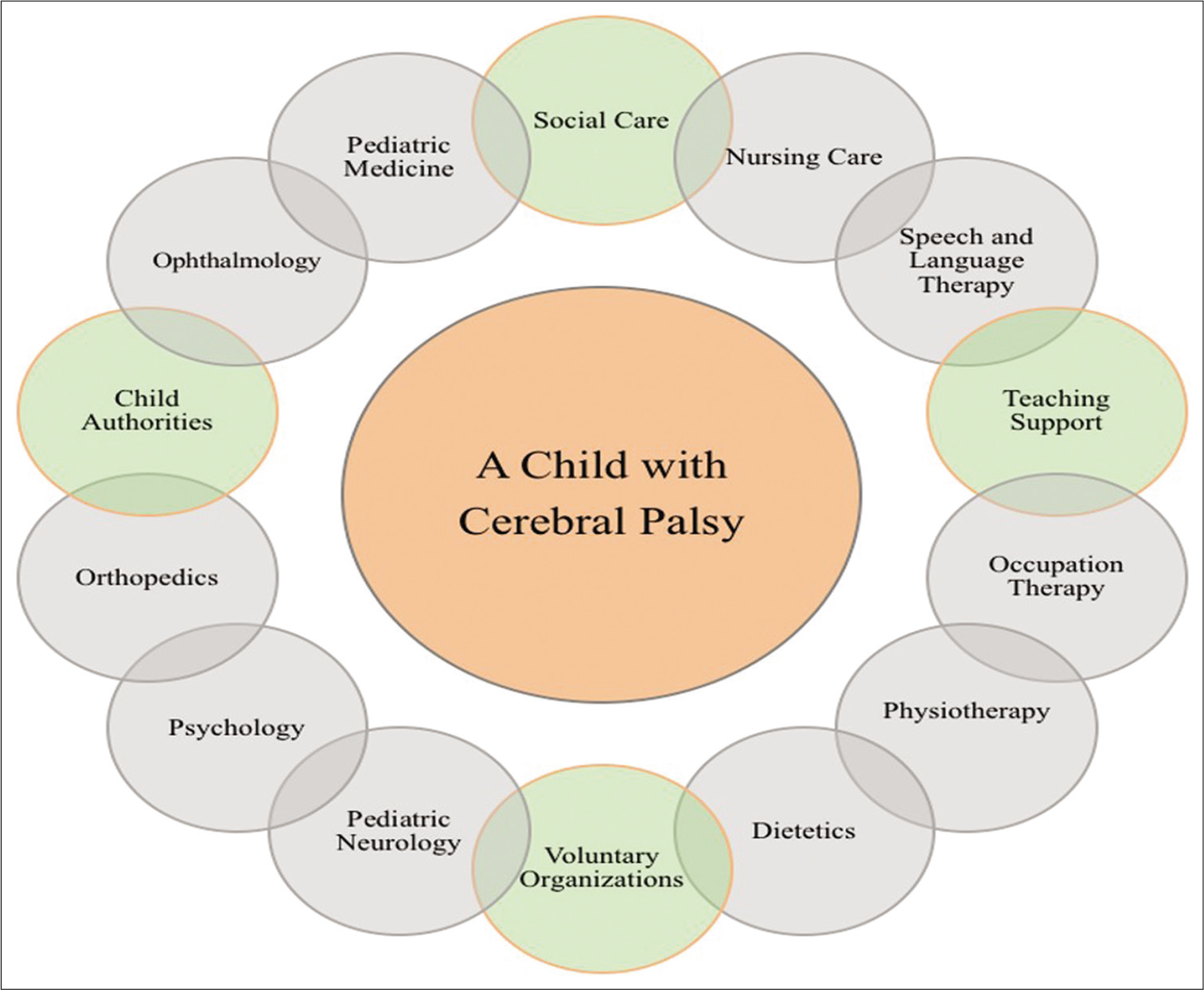
- Multidisciplinary team for the child with cerebral palsy. Adapted from NICE guidelines.
Evidence based management of CP
In the last decade, the CP evidence base has rapidly expanded, providing clinicians and families with the possibility of newer, safer, and more effective interventions. In 2013, Novak et al.[15,16] conducted a systematic review of interventions for children with CP and color-coded the evidence using Evidence Alert Traffic Light Grading System. Where green = go (high-quality evidence indicates effectiveness); red = stop (high-quality evidence indicates ineffectiveness); and yellow = measure individual outcomes (evidence is conflicting).
CP and comorbidities
As already discussed, all commodities should be recognized, and specific treatments [Table 8] for these problems should be managed. This can substantially improve the child’s outcome and quality of life, even though CP itself cannot be treated.
| Comorbidity | Incidence (%) | Evidence-based intervention/prognosis |
|---|---|---|
| Pain | 75 | Treat to prevent sleep and behavioral disorders |
| Intellectual disability | 50 | Poorer for ambulation, continence, academics |
| Non-ambulant | 33 | Independent sitting at 2 years predicts walking |
| Hip dislocation | 33 | 6–12 monthly X-ray of pelvis |
| Non-verbal | 25 | Augment speech therapy |
| Epilepsy | 25 | Antiseizure medications |
| Behavior disorder | 25 | Detect early and should be managed |
| Bladder incontinence | 25 | Investigate and allow time |
| Sleep disorder | 20 | Investigate and manage early |
| Blindness | 10 | Assess early and vision therapy |
| Non-oral feeding | 7 | Allow swallow safety and monitor growth |
| Deafness | 4 | Assess early and hearing aid |
[Figure 3a] shows child with diplegic cerebral palsy, [Figure 3b] shows MRI of brain with periventricular leukomalacia commonly seen in diplegic cerebral palsy. [Figure 3c] clinical photo showing microcephaly in a child with quadriplegic CP with multicystic encephalomalacia with subdural effusion on MRI of brain [Figure 3d]. [Figures 4a and b] shows child with dyskinetic cerebral palsy with MRI (4b) brain T2 axial showing hyperintensities in bilateral posterior putamen and thalami suggestive of acute hypoxic insult. [Figures 4c and d] shows child with dyskinetic cerebral palsy with dystonic posturing and arching of neck due to dystonia and MRI brain (4d) T2 axial showing bilateral symmetrical hyperintensities in globus pallidus due to bilirubin induced neurologic dysfunction. [Figures 5a and b] shows child with dystonic cerebral palsy with severe arching of back and neck due to dystonia and computed tomography (b) brain showing bilateral thalamic calcifications with cerebral atrophy with enlarged ventricles suggestive of hypoxic insult. [Figures 5c and d] shows child with hemiplegic cerebral palsy with magnetic resonance imaging (d) of brain showing porencephalic cyst. [Figures 6a-c] perisylvian syndrome- type of cerebral palsy showing drooling, MRI of brain (6b, c) showing gliosis in the perisylvian region. [Figures 6d-f] neonatal hypoglycemic brain injury: Clinical photo (6d) showing strabismus and MRI of brain: DWI (6e) and ADC (6f) showing restricted diffusion and low ADC in the bilateral parieto-occipital region.
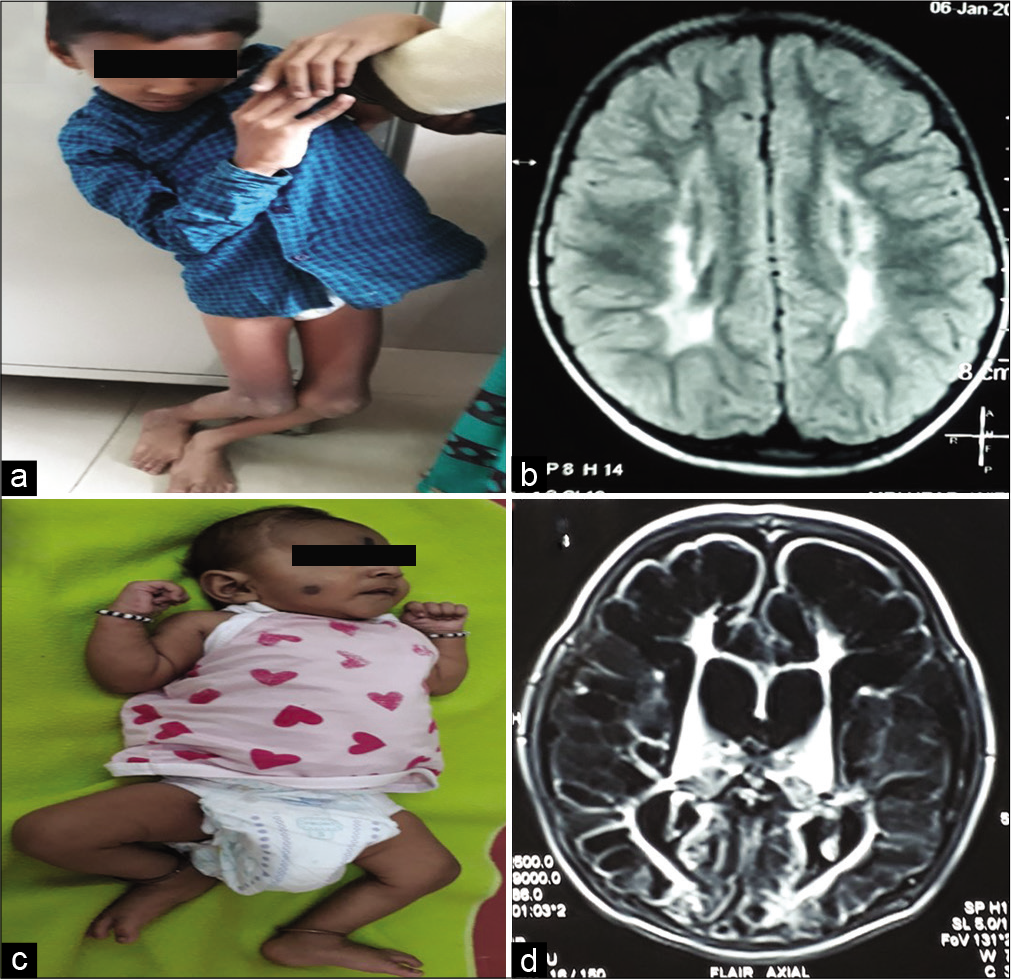
- (a) Bilateral spastic cerebral palsy – spastic diplegic type with scissoring of both lower limbs with deformity. (b) Magnetic resonance imaging (MRI) of brain T2 axial sections showing periventricular hyperintensities suggestive of periventricular leukomalacia. (c) Clinical photo showing microcephaly in a child with bilateral spastic cerebral palsy of quadriplegic type with multicystic encephalomalacia with subdural effusion on MRI of brain (d).
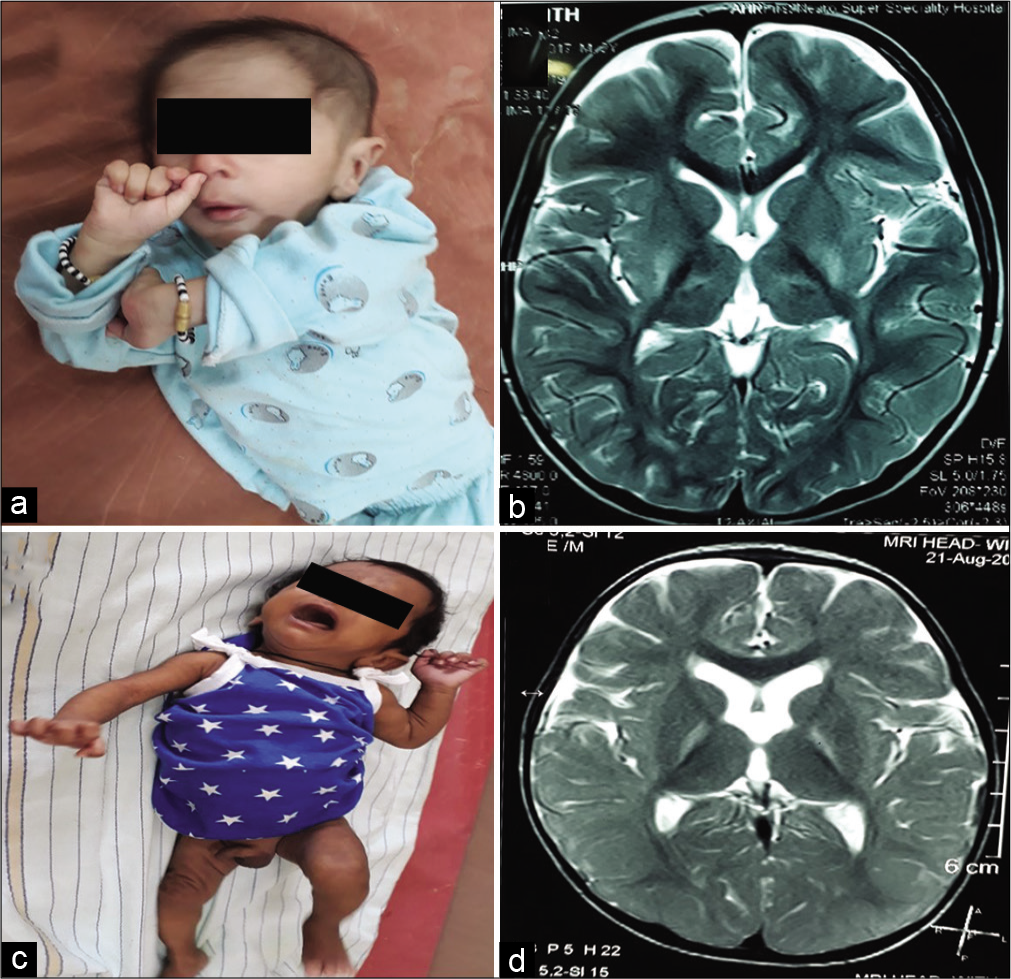
- (a and b) Child with dyskinetic cerebral palsy with magnetic resonance imaging (MRI) (b) brain T2 axial showing hyperintensities in bilateral posterior putamen and thalami suggestive of acute hypoxic insult. (c and d): Child with dyskinetic cerebral palsy with dystonic posturing and arching of neck due to dystonia and MRI brain (d) T2 axial showing bilateral symmetrical hyperintensities in globus pallidus due to bilirubin-induced neurologic dysfunction.
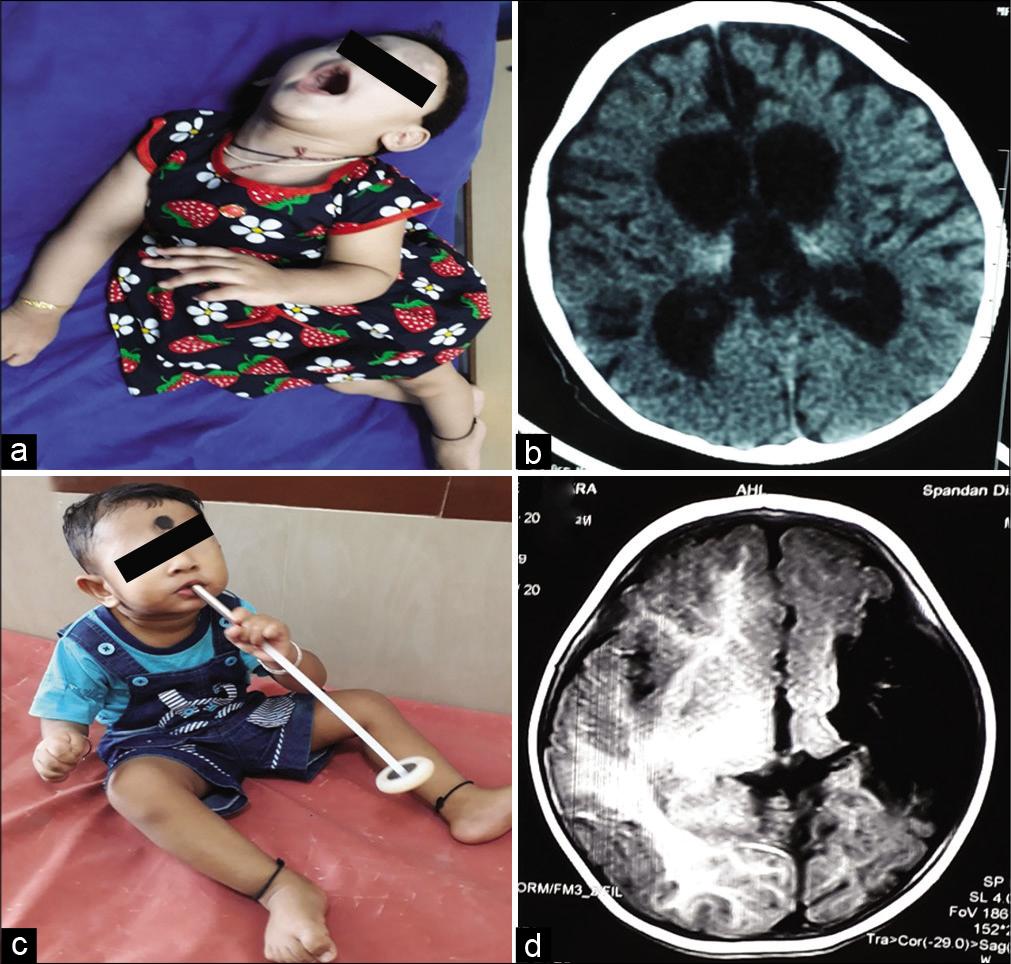
- (a and b) Child with dystonic cerebral palsy with severe arching of back and neck due to dystonia and computed tomography (b) brain showing bilateral thalamic calcifications with cerebral atrophy with enlarged ventricles suggestive of hypoxic insult. (c and d): Child with hemiplegic cerebral palsy with magnetic resonance imaging (d) of brain showing porencephalic cyst.
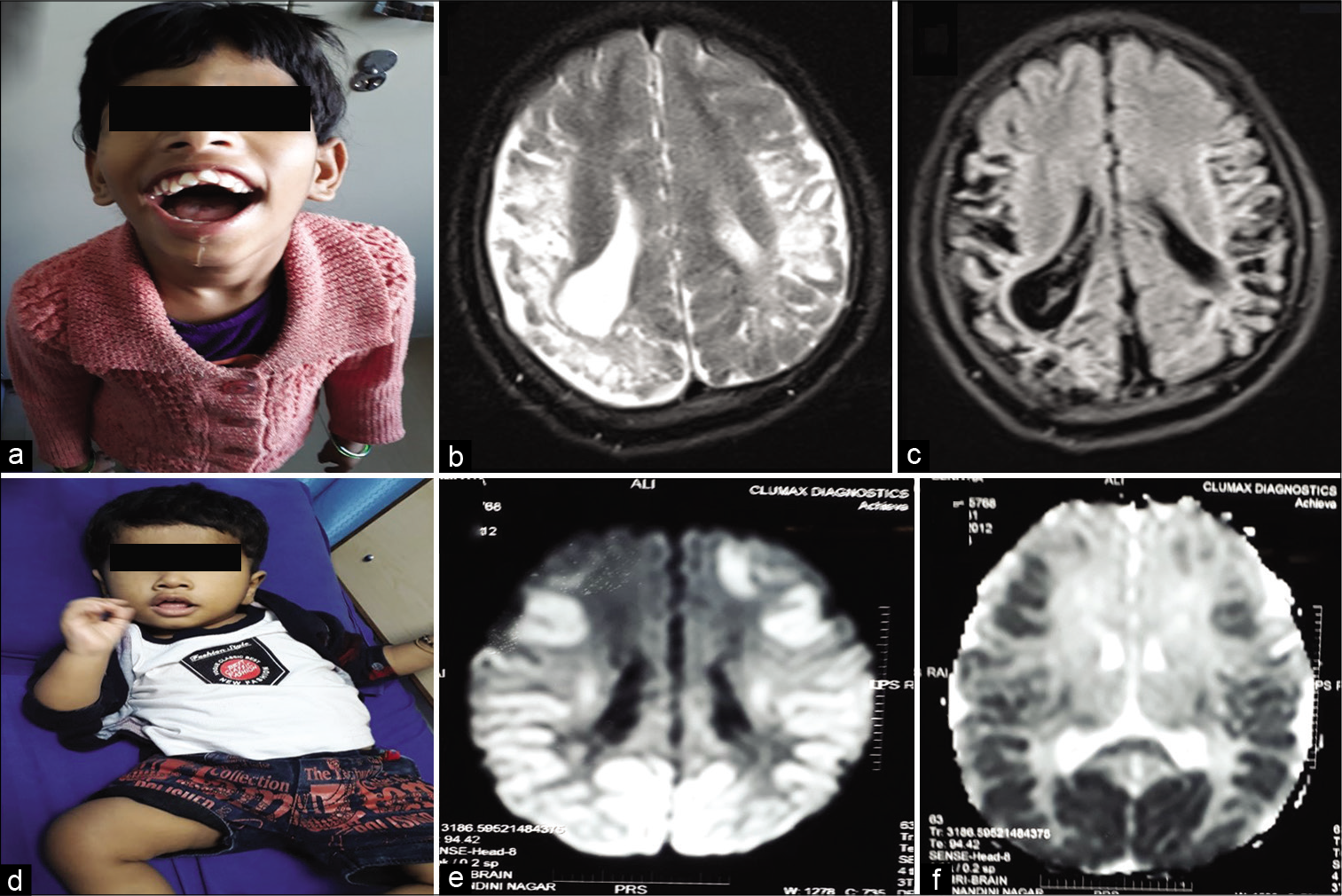
- (a-c) Perisylvian syndrome – type of cerebral palsy showing drooling, magnetic resonance imaging (MRI) of brain (b and c) showing gliosis in the perisylvian region. (d-f): Neonatal hypoglycemic brain injury: Clinical photo (d) showing strabismus and MRI of brain: DWI (e) and ADC (f) showing restricted diffusion and low ADC in the bilateral parieto-occipital region.
Medical management of CP
Wide assortments of medications are used in CP to reduce symptoms and address complications and treat comorbidities. Children who experience spasticity and unwanted or uncontrolled involuntary movements such as dystonia, chorea, and athetosis are often prescribed drugs to minimize the movements, relax muscles, increase comfort, and facilitate better posture and functionality. Drug therapy is also used to treat seizures, behavioral issues, pain, bowel movements, and manage other comorbidities and improve quality of life.
Spasticity management
Spasticity treatment may include one or more of the following options:
Oral medications
Chemical blockage: Botulinum toxin and/or phenol
Intrathecal baclofen pump
Surgical management
Physical measures such as physiotherapy, occupational therapy, orthosis, and plaster caster use.
The most commonly used drugs and dosages are:
Baclofen – dose 0.12–1 mg/kg/day
Tizanidine – 0.3 mg–0.5 mg/kg/day
Benzodiazepines (e.g., diazepam – 0.12–0.8 mg/kg/day and clonazepam – 0.01–005 mg/kg/day)
Dantrolene sodium: 3–12 mg/kg/day.
FEW TIPS TO SELECT DRUGS FOR SPASTICITY
Intractable seizures AND seizure tendency – avoid baclofen
Spasticity AND dystonia – baclofen
Sleep problems – bedtime diazepam/tizanidine
Myoclonus – clonazepam
Liver problems – avoid tizanidine, dantrolene.
MANAGEMENT OF MOVEMENT DISORDERS IN CP
Medications used for dystonia are:
Trihexyphenidyl – Anticholinergic. Starting dose of 0.1–0.2 mg/kg/day, increase once in 3 days to the maximum dose of 1 mg/kg/day (total-max dose <10 kg–30 mg/day and more than 10 kg–60 mg/day. can be tried with monitoring adverse effects). The main side effects are dry eyes and mouth, gastrointestinal disturbances, urinary retention, and behavioral disturbances
Tetrabenazine – dose 0.5 mg–4 mg/kg/day. In 2 or 3 divided doses, increase once in 3 days. Side effects include drowsiness, parkinsonism, depression, insomnia, nervousness, anxiety, and akathisia
Baclofen (in high doses 1 mg/kg /day reduces dystonia)
Levodopa (Syndopa) – start at 0.5 mg/kg/day up to 10– 20 mg/kg/day)
Benzodiazepines (e.g., diazepam – 0.12–0.8 mg/kg/day and clonazepam – 0.01–005 mg/kg/day)
Deep brain stimulation.
REHABILITATION: PHYSIOTHERAPY AND OCCUPATIONAL THERAPY
Therapy program
Infant-stimulating advanced postural equilibrium and balance reactions to provide head and trunk control
Toddler and preschool-stretching the spastic muscles strengthening the weak ones and promoting mobility
Adolescent-improving cardiovascular status.
Therapy methods
Bobath neurodevelopmental therapy. This is the most commonly used therapy method in CP world-wide. The aims of this therapy are to normalize muscle tone, stimulate normal movements, and inhibit abnormal primitive reflexes. It uses reflex inhibitory positions to decrease tone and promote the development of advanced postural reactions by stimulating key points of control.
Hand-arm bimanual intensive training (HABIT) for hemiplegic CP where the child is trained to use both hands together through repetitive tasks such as drumming, pushing a rolling pin, and pulling apart construction toys (Legos).
Constraint-induced movement therapy (CIMT) involves restraint of the unaffected limb to encourage the use of affected limb during the therapeutic tasks. The restraint may be by the use of casting or physically restraining by holding the normal hand.
Context-focused therapy involves changing the environment rather than the child’s approach.
Goal-directed functional training lays emphasis on activities based on goals set by the child using a motor learning approach.
OCCUPATIONAL THERAPY IN CP
As CP can affect children in very different ways, the occupational therapist will start with a full assessment. The focus of the assessment will be as much about understanding the child’s abilities as understanding what they are finding difficult and why. During the assessment, the occupational therapist will also want to gain an understanding of the child’s own goals as well as the goals of their parents, carers, or school. The occupational therapist will provide tailored advice once information obtained during assessment. Below are some examples of how an occupational therapist can assist:
Improve the child’s skills by adapting tasks, teaching, and training or advise on appropriate assistive technology to maximize independence and increase participation
Provides structural building changes and/or equipment in home and schools to facilitate safe access
Facilitate access to the school curriculum and support school staff in understanding how to best support the child’s education
Provides advice on equipment and techniques to maintain postural alignment, to reduce the risk of fixed postural changes such as splinting, supportive seating, and positioning while sleeping.
EXERCISES USED IN OCCUPATIONAL THERAPY
Occupational therapy involves using functional activities to progressively improve functional performance. Occupational therapy exercises focus on the following skill areas:
Fine motor control – improves hand dexterity by working on hand muscle strength, finger isolations, in-hand manipulations, arching the palm of the hand, thumb opposition, and pincer grasp. Activities include squeezing a clothespin, playing with water squirt toys, and pushing coins into the slot of a piggy bank
HABIT: Bilateral coordination
Upper body strength and stability – play focuses on strengthening and stabilizing the trunk (core), shoulder and wrist muscles through exercises such as crawling and lying on the prone position while reading
Crossing the midline – these activities such as making figure eights with streamers and throwing balls at a target to the right or left of center, teach the child to reach across the middle of their body with their arms and legs to the opposite side
To improve visual motor skills, activities that improve hand-eye coordination such as drawing, stringing beads, catching, and throwing a ball
For visual perception – activities include alphabet puzzles, playing with different shapes, and matching games
For self-care, activities such as brushing their teeth, getting dressed, and self-feeding are useful.
VARIOUS TECHNIQUES TO REACH THEIR GOALS ARE
Pediatric CIMT – ask the child to use weaker limb while restraining normal limb
Sensory integration therapy – here advise activities that stimulate various sensations such as the skin by providing different texture experiences; sand, water, dough, and finger painting.
ROLE OF BOTULINUM TOXIN AND ORTHOPEDIC INTERVENTION IN CP[17-19]
Quick orthopedic examination includes
Gait and gross motor function classification system (GMFCS) grading
Analysis of range of motion and joint contractures of various joints
Motor strength assessment
Assessment of torsional deformity
Upper limb and spine examination.
PATIENT SELECTION FOR BOTULINUM TOXIN
-
Favorable factors
Focal goals with specific anticipated functional benefits
Increased dynamic muscle stiffness
Muscular hypertonia with a functional goal.
-
Negative factors
Severe fixed contractures
Bony torsion and joint instability
Bleeding disorders
Too many target muscles – consider other treatment options, or prioritize.
TIMING OF TREATMENT FOR BOTULINUM TOXIN
For the lower extremity, early treatment is preferable: 1–6 years of age
For the upper extremity: More than 4 years of age
Treatment during the dynamic phase of motor development maximizes the chance of permanent modification of the disease
Early treatment may allow postponement, simplification or even, occasionally, avoidance of surgery
Later treatment can still be valuable in terms of pain relief, ease of care, and functional goals such as sitting or standing.
RECOMMENDED SAFE DOSE OF BOTULINUM TOXIN
Range (U/Kg body wt.): 1–20 U
Maximum total dose (U): 400 U
Range maximum dose/site (U): 10–50 U.
We did study Koushik et al.[20] there is no difference in outcome with the administration of injection botulinum toxin manual versus ultrasound-guided for lower limb muscle spasticity.
ORTHOPEDIC SURGERIES IN CP
Various surgeries done
-
To improve muscular problems
Tendon lengthening: Tendoachilles lengthening and hamstring lengthening
Intramuscular/fractional lengthening: Gastrocnemius/hamstring fractional lengthening
Muscle release: Hamstring, iliopsoas brim release, and adductor tenotomy
Tendon transfer: Pronator rerouting, split posterior tibial/tibialis anterior transfers, and semitendinosus transfer
Neurectomy: Obturator neurectomy.
-
To improve static problems
Reduce subluxated or dislocated joints: Hip varus derotation osteotomy, acetabular surgeries for coverage (shelf osteotomy), and excisional arthroplasty
Correction of bony abnormalities and rotational problems: Femoral shortening/extension/derotation osteotomy, tibial corrective osteotomy, and foot lateral column lengthening surgery
Fuse joints to provide stability: Triple arthrodesis of foot, etc.
Spine deformity correction surgery
We reported earlier, Gowda et al.,[21] that hip dysplasia is not uncommon in Indian children with CP.
CEREBRAL VISUAL IMPAIRMENT (CVI)
CVI is defined as visual loss resulting from damage to the retrochiasmatic visual pathways and cerebral structures. The eye and anterior pathways (optic nerve and chiasma) are essentially normal and do not contribute to the visual impairment. The term “Cerebral” is used as there is the involvement of the sub-cortical structures, white matter of the brain, and visual processing areas also in this process, in addition to the visual cortex. It should be differentiated from autism spectrum disorder, severe intellectual disability, and delayed visual maturation in infants.
ENVIRONMENTAL MODIFICATION FOR TREATMENT OF CVI
Reducing clutter – minimize the number of objects in the working space/play area to avoid visual confusion
Increasing lighting and contrast – use dark pencils, outline pictures, add a table lamp, etc.
Presenting tasks in the preferred field of gaze
Encouraging auditory learning
Using touch to identify objects
Marking the edges of steps and pathways in contrasting colors to delineate the path clearly.
PERISYLVIAN SYNDROME AND MANAGEMENT OF DROOLING IN CP
Perisylvian syndrome, also called Worster Drought Syndrome or Congenital Bilateral Perisylvian syndrome, is quite a common but under-recognized and sub-optimally managed entity. It falls within the spectrum of CP and usually has a predominantly motor component, but it can also have cognitive, behavioral issues, and epilepsy as comorbid conditions. All these complaints can be localized to the involvement of Perisylvian area. The specialty of this entity is that the motor impairment is only pseudobulbar paresis with mild spastic quadriplegia, thus making the patient have a good GMFCS score. However, the speech and feeding problems are severe and if they are not addressed, they lead to various complications which will hamper the quality of life of the patient. All we have to understand is that these children have a specific phenotype which when recognized early, can make a significant difference in management and prognosis.
MANAGEMENT OF DROOLING[9]
It is a challenging condition and requires the coordinated services of Pediatrician, Pediatric Neurologist, Speech therapist, ENT Surgeon, and Occupational Therapist. There are two main approaches:
Non-invasive – oral motor therapy and pharmacological therapy
Invasive – Surgery – rarely used.
NON-INVASIVE MODALITIES
Positioning
When seated, a child should be fully supported and comfortable. Good posture with proper trunk and head control with appropriate seating devices facilitates better control of drooling and swallowing.
Feeding skills
Poor feeding skills can exaggerate drooling. Care should be taken to ensure lip closure, tongue movements, and swallowing properly. Avoidance of acidic fruits is worthwhile.
Oral facial facilitation
Most widely used and first line of therapy. This improves oromotor control, sensory awareness, and frequency of swallowing, done by a speech therapist. It is easy to do with no side effects, but may only have a short-term benefit.
Icing, effect lasts for 5–30 min, improves tone, swallow reflex
Brushing, effect lasts for 20–30 min, to be done before meals
Vibration improves tone in high tone muscles
Manipulation such as tapping, stroking, and patting, firm pressure directly to muscles using fingertips improves oral awareness
Oral motor sensory exercise, lip and tongue exercises.
Oral prosthetic devices such as chin cup, dental appliances for mandibular stability, better lip closure, tongue position, and swallowing.
Pharmacological is the second line of management. Anticholinergic drugs such as atropine, benztropine, glycopyrrolate, scopolamine, and benzhexol hydrochloride work by anticholinergic blockade of muscarinic receptor sites to reduce parasympathetic stimulation of salivary glands. However, they also act on muscarinic receptors elsewhere too causing side effects. They are quite effective, but owing to these side effects, they are not considered very ideal. However, these effects are reversible after the stoppage.
In conclusion, mild drooling can be managed by behavioral strategies, hands-on therapies, and proper positioning. In persistent problematic drooling, medications may be tried, but if they still do not respond, surgical interventions may be tried. Finally, a coordinated interdisciplinary approach may alleviate this complex issue.
FEEDING PROBLEMS IN CP
Recent systemic review and meta-analyses by Speyer et al. showed that pooled prevalence of 44% for drooling, 50% for swallowing problems, and 54% for feeding problems in children with CP.[22] The feeding problems are very common in children with CP. A thorough nutritional assessment should be done, and nutritional support should be started with dietary advice and modification of oral feeding, if safe and acceptable. In the presence of unsafe swallowing and inadequate oral intake, enteral nutrition should be initiated, and early gastrostomy placement should be evaluated and discussed with parents/caregivers. Gastrointestinal problems in CP children are frequent, should be actively detected and appropriately managed to prevent nutritionals status of child.[23] Various gastrointestinal problems are oromotor dysfunction, GERD, and constipation.[23]
PROGNOSIS OF CP
Prognosis regarding walking
A common question asked by parents of children with CP is whether the child will be able to walk independently?
In general, children have an enhanced capacity for brain plasticity, resulting in a capacity to recover and improve from brain insults.[24]
-
The prognosis depends on the type and extent of brain injury. The more severe the child’s physical, functional, or cognitive impairment, the greater the possibility of difficulties with walking.[14]
The ability to sit independently and rollover at 2 years of age is predictive of future ambulation[25]
If a child can sit at 2 years of age, it is likely, but not certain, that they will be able to walk unaided by 6 years of age[14]
If a child cannot sit but can roll at 2 years of age, there is a possibility that they may be able to walk unaided by 6 years of age[14]
If a child cannot sit or roll at 2 years of age, they are unlikely to be able to walk unaided[14]
General rule: Children with independent sitting by 2 years walk, those who are unable to sit by 4 years of age rarely walk[26]
The type of CP further adds additional prognostic information as per available evidence.
Most children with hemiplegic CP will be able to ambulate independently. Usually, they walk by 2 years of life without any other major comorbidities[25,26]
More than 50% of spastic diplegia learn to walk[26]
Spastic quadriplegic CP, only 33% usually walk (mostly after 3 years) and 25 usually required completed total care[26]
Dyskinetic CP has an intermediate chance of walking.[26]
Poor prognostic factors for walking, in general, are bilateral spastic and dyskinetic CP, IQ <50, severe visual impairment, active epilepsy, absence of rolling over/sitting/crawling at 2 years of age, absence of functional hand use by 2 years, the persistence of primitive reflexes beyond 2 years of life, and GMFCS class IV to V. [Table 9] shows, the prognosis of CP based on MRI of brain.[27]
| Type of involvement | Minimal | Moderate | Severe |
|---|---|---|---|
| Prognosis based on basal ganglia involvement | |||
| Radiological description and involvement | Discrete lesions in posterior part of putamen | Marked focal lesions in posterior putamen with thalamus, usually have equivocal or abnormal posterior limb of internal capsule (PLIC) | Marked diffuse involvement of the posterior putamen and thalami with completely absent signal from myelin in the PLIC |
| Prognosis (at school age) | 1.Dyskinetic or Athetoid CP 2.Minor neuromotor abnormality 3.Normal cognitive development |
1.Dyskinetic/spastic CP 2.Microcephaly 3.Severe GDD |
|
| Prognosis based on white matter changes | |||
| Radiological description and involvement | Mild discrete periventricular white matter changes only | Focal abnormalities in the white matter with or without cortical involvement | Diffuse and extensive signal changes throughout the white matter |
| Prognosis (at school age) | Normal | Normal or only minor motor abnormalities, such as poor hand function and balance | Microcephaly, GDD, walking with without support, spastic diplegia or quadriparesis, and poor perceptual-motor abilities |
MRI: Magnetic resonance imaging, GDD: Global developmental delay
A study done by us, Surender et al.,[28] on caregiver-reported health-related quality of life (HRQOL) of children with CP and their families and its association with gross motor function. HRQOL in CP and their caregivers is highly impaired. The degree of impairment is associated with physical independence, mobility, clinical burden, and social integration dimensions.
PREVENTION OF CP
Primary prevention – preventing the occurrence of CP
-
1. Health promotion
Health education for adolescent girls and improving anemia and nutrition
Improvement on the nutritional status of the community
Improvement in pre-natal, natal, and post-natal care
Optimum health-care facility and infrastructure
Awareness regarding developmentally supportive neonatal care.
-
Specific protection
Rubella immunization for girls
Folic acid supplementation during pregnancy
Universal iodization of salt
Prevention of exposure to teratogenic agents and radiation
Pre-natal tests such as triple test and quadruple test
Universal immunization for all children
Administering anti-D globulin to prevent Rh- isoimmunization
Intrapartum fetal monitoring to detect fetal distress
Improving immunization coverage and preventing accidents.
Secondary prevention
Halting and arresting disease progression by early diagnosis and treatment
Newborn thyroid screening
Neonatal metabolic screening for a treatable inborn error of metabolisms such as galactosemia and phenylketonuria
High-risk newborn follow-up clinics for early detection “at risk babies”
Cervical encerlage for cervical incompetence to prevent prematurity
Antenatal administration of magnesium sulfate to mothers at risk of preterm delivery before 34 weeks of gestation reduces the risk of CP[29]
Therapeutic hypothermia for neonates with hypoxic- ischemic encephalopathy.[30]
Tertiary prevention
Tertiary prevention is by preventing complications and maximization of functions by disability limitations and rehabilitation.
Assistive technology by equipment or ambulatory devices to improve independence, for example, walking frames, wheelchairs, etc.
Administration of botulinum toxin and giving anti- spasticity medicines to reduce spasticity
Refractory error correction and vision stimulation and rehabilitation
Communication skills may be enhanced by the use of bliss symbols, talking typewriters, electronic speech- generating devices, hearing aids, etc.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Proposed definition and classification of cerebral palsy, April 2005 executive committee for the definition of cerebral palsy. Dev Med Child Neurol. 2005;47:571-6.
- [CrossRef] [PubMed] [Google Scholar]
- Surveillance of cerebral palsy in Europe: A collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2007;42:816-24.
- [CrossRef] [Google Scholar]
- Etiological factors in cerebral palsy: An historical review. Dev Med Child Neurol. 1981;23:633-42.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile, predisposing factors, and associated co-morbidities of children with cerebral palsy in South India. J Pediatr Neurosci. 2015;10:108-13.
- [CrossRef] [PubMed] [Google Scholar]
- Surveillance of cerebral palsy in Europe: Reference and training manual. Med Educ. 2009;43:495-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral palsy: Not always what it seems. Arch Dis Child. 2001;85:356-60.
- [CrossRef] [PubMed] [Google Scholar]
- Implementation of the hammersmith infant neurological examination in a high-risk infant follow-up program. Pediatr Neurol. 2016;65:31-8.
- [CrossRef] [PubMed] [Google Scholar]
- Practice parameter: Diagnostic assessment of the child with cerebral palsy: Report of the quality standards subcommittee of the American academy of neurology and the practice committee of the child neurology society. Neurology. 2004;62:851-63.
- [CrossRef] [PubMed] [Google Scholar]
- Heterogeneity in cerebral palsy: Variation in neurology, comorbidity and associated conditions In: Bax M, Gillberg C, eds. Comorbidities in Developmental Disorders. London: Mac Keith Press; 2011. p. :20-39.
- [Google Scholar]
- Risk factors associated with epilepsy development in children with cerebral palsy. Childs Nerv Syst. 2019;35:1181-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral Palsy in Under 25s: Assessment and Management Israel: National Institute for Health and Care Excellence; 2017.
- [Google Scholar]
- State of the evidence: Systematic review of interventions for children with cerebral palsy. Dev Med Child Neurol. 2013;55:885-910.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence-based diagnosis, health care, and rehabilitation for children with cerebral palsy. J Child Neurol. 2014;29:1141-56.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendations for the use of botulinum toxin Type A in the management of cerebral palsy. Gait Posture. 2000;11:67-79.
- [CrossRef] [Google Scholar]
- Best clinical practice in botulinum toxin treatment for children with cerebral palsy. Toxins. 2015;7:1629-48.
- [CrossRef] [PubMed] [Google Scholar]
- Orthopedic surgery in cerebral palsy: Instructional course lecture. Indian J Orthop. 2017;51:240.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized control trial of botulinum toxin a administration under ultrasound guidance against manual palpation in spastic cerebral palsy. J Pediatr Neurosci. 2018;13:443-7.
- [CrossRef] [PubMed] [Google Scholar]
- Developmental dysplasia of spastic hip in children with cerebral palsy in Southern India. Indian Pediatr. 2016;53:259-60.
- [Google Scholar]
- Prevalence of drooling, swallowing, and feeding problems in cerebral palsy across the lifespan: A systematic review and meta-analyses. Dev Med Child Neurol. 2019;61:1249-58.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation and treatment of malnutrition and associated gastrointestinal complications in children with cerebral palsy. Pediatr Gastroenterol Hepatol Nutr. 2019;22:122-31.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral palsy: Comprehensive review and update. Ann Saudi Med. 2006;26:123-32.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral Palsy and Other Neurodevelopmental Disorders. Available from: https://www.pedneuroaiims.chalopadho.com/s/classroom/1/chapter/7 [Last accessed on 2020 Jan 09]
- [Google Scholar]
- Neonatal brain MRI and motor outcome at school age in children with neonatal encephalopathy: A review of personal experience. Neural Plast. 2003;10:51-7.
- [CrossRef] [PubMed] [Google Scholar]
- Caregiver-reported health-related quality of life of children with cerebral palsy and their families and its association with gross motor function: A South Indian study. J Neurosci Rural Pract. 2016;7:223-7.
- [CrossRef] [PubMed] [Google Scholar]
- Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks' gestation: A systematic review and meta-analysis. Am J Obstet Gynecol. 2009;200:595-609.
- [CrossRef] [PubMed] [Google Scholar]
- Cooling for newborns with hypoxic ischaemic encephalopathy. Evid Based Child Health. 2008;3:1049-115.
- [CrossRef] [Google Scholar]






