Translate this page into:
Vitamin-D status and bone mineral density in asthmatic children on long-term inhaled corticosteroids
*Corresponding author: M. R. Savitha, Department of Pediatrics, Mysore Medical College and Research Institute, Mysore, Karnataka, India. drsavithamr@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Thanuja B, Savitha MR. Vitamin-D status and bone mineral density in asthmatic children on long-term inhaled corticosteroids. Karnataka Paediatr J 2020;35(1):39-47.
Abstract
Asthma is the most common chronic respiratory illness affecting children. Inhaled corticosteroids (ICS) form the main treatment modality in asthma. Reduction in bone mineral density (BMD) is an important adverse effect of steroid usage. This side effect is an established entity with oral corticosteroids but minimal with ICS therapy. However, there are reports regarding the detrimental effect of chronic therapy with ICS. Long-term high-dose budesonide more than 800 μg/day has been shown to reduce the BMD. However, this effect was not consistently seen with moderate doses of 400–800 μg/day. Anticipating the impact of steroids on bone metabolism and monitoring for it is essential. Annual monitoring of Vitamin-D levels and BMD in children on chronic therapy is beneficial for the early detection and management of steroid-induced osteopenia. Judicious ICS use at the lowest effective dose should be tailor-made for every individual.
Keywords
Asthma
Bone mineral density
Inhaled steroids
Vitamin-D
INTRODUCTION
Asthma, the most common chronic respiratory illness affecting children worldwide is defined by the Global Initiative for asthma as: “History of respiratory symptoms such as wheeze, shortness of breath, chest tightness, and cough that varies over time and in intensity along with variable expiratory airflow limitation.”[1] In 2016, the Global Burden of Disease study gave a worldwide asthma estimate of 339.4 million, which represents a 3.6% increase in age-standardized prevalence since 2006.[2] About 10% of the asthmatics are from the Indian subcontinent.[3] The age-wise global prevalence of asthma, according to CDC, is 8.6% in less than18 years, 10.6% in 5–11 years, and 9.7% in 12–17 years age-group.[4] According to the International Study of Asthma and Allergies in Childhood Phase 3, there is variation in asthma prevalence: 2.4–37.6% in 6–7 years old and 0.8–32.6% in 13–14 years old children.[5] There is rising trend in India from 5% in 2002 to 7.3% in 2008 and 10.3% in 2010.[6-8]
Glucocorticoid utility in childhood is to the tune of 10%, and the largest contribution to chronic- steroid exposure in children is by inhaled corticosteroid (ICS).[9] ICS is the most effective controller drugs with multiple mechanisms of action: Anti-inflammatory, reduction of airway responsiveness, reversal of β2 receptor downregulation, and prevention of airway remodeling.[1,10] Benefits are seen within 2–3 weeks of starting therapy.[10] There have been widespread, long- standing concerns regarding adverse-events such as decreased bone mineral density (BMD), fractures, and reduction of growth in children with corticosteroid usage.[11] High-dose ICS and oral corticosteroid (OCS) can be associated with decreased BMD.[12,13] Some studies have shown no detrimental effects of ICS on BMD.[14,15]
There continues to be conflicting evidence regarding the effect of inhaled steroids on BMD.[14-21] The aim of our article is to review the effect of ICS on Vitamin D and its interplay with BMD in asthmatic children.
ICS IN ASTHMA
Many factors and comorbidities affect the severity of asthma and its control. Failing to provide the deemed attention to these factors can lead to poor control and poor quality of life. In sensitized individuals, reduction or elimination of food and aero-allergens is necessary for adequate asthma control along with pharmacological therapy.[22] The common pathophysiological mechanism of inflammation and atopy makes the clinicians to use controller medications and hence ICS is one of the important components of asthma therapy since its introduction in the 80s. ICS is very effective in childhood asthma and is superior to other drugs used.[23] Even low-dose of ICS is known to reduce mortality in asthma. The benefits of long-term therapy are emphasized in national and international guidelines.[1,10]
Responsiveness to therapy varies, and it refers to how easily asthma-control can be achieved with treatment. Based on the management patterns, asthma can be classified as: Easy- to-control, difficult-to-control, exacerbators, and refractory (poorly controlled in spite of multiple and high-dose ICS).[22]
Controllers are used to reduce inflammation of airway, symptoms, exacerbations, and decline in pulmonary function. In mild asthma, low-dose ICS-formoterol can be taken as needed to reduce the risk of exacerbations.[1] Best outcomes are obtained when ICS is initiated soon after the diagnosis of asthma because:
Early initiation of low-dose ICS provides an improvement in pulmonary function
Severe exacerbation in those not on ICS have a greater long-term decline in pulmonary function[24]
In occupational asthma, early removal of sensitizing agent exposure and initiation of controller treatment increases the likelihood of recovery.[25]
Systemic steroids are important in hastening the recovery from acute exacerbations and also preventing replase. They are used in all but the mildest exacerbations, preferably administered within 1 hour of presentation.[26]
It is particularly important if:
Initial treatment with SABA (short-acting beta-2 agonist) fails to achieve improvement
Exacerbation developed while on OCS
Previous history of exacerbations requiring OCS.
Route of delivery in acute exacerbations: Oral route is as effective as intravenous. The oral route is preferred as it is less invasive, quicker, and cheaper. Liquid formulation enables easier dosage adjustment and administration. OCS requires a minimum 4 hours to produce clinical improvement. Intravenous corticosteroids are preferred if patients are dyspneic, unable to swallow, vomiting, or when they require ventilation.[27]
A Cochrane review (six trials; 374 patients) found that the use of corticosteroids was associated with a significant reduction in the relapse rate, hospitalization rate, and SABA use.[28] In another review of 12 studies involving 863 patients, corticosteroid administration within 1 hour of presentation significantly reduced hospital admission rates with no increase in side effects.[29] A 5–7 days short-course of OCS is as effective as 10–14 days therapy.[30] In patients receiving systemic steroids, tapering is not needed if the duration of treatment is less than 2 weeks.[31]
In acute exacerbations, ICS was associated with lesser hospital admissions in patients with mild-moderate exacerbations. However, in combination with systemic corticosteroids, ICS provides no additional benefits.[32-34] Patients who are already on ICS should continue their medication during the acute attack.[35]
ICS is the controllers of choice for stable asthma management.[33]
All ICS is efficacious when used in equipotent doses
Most benefits are obtained at low-moderate doses. Increasing the dose benefits only a minority
ICS should be started and used at the lowest possible dose
High-dose ICS should be avoided to reduce the side effects.
NORMAL BONE FORMATION AND ROLE OF VITAMIN-D
Bone is a metabolically active rigid organ undergoing constant modeling and remodeling. It is the major reserve of calcium, phosphorus, and magnesium.[36] The bone composition is as follows: 50–70% minerals, 20–40% organic matrix, 5–10% water, and less than 3% lipids. The major mineral content is calcium-hydroxyapatite found deep in the bone matrix; the surface coating of remodeled bone is by amorphous calcium- phosphate. Calcium and phosphate binding-proteins regulate the formation of hydroxyapatite crystals and lead to mineral deposition in an orderly manner.[37]
Osteoprogenitor cells originate from pluripotent stem cells. The new bone matrix is synthesized by osteoblasts and supported by osteocytes. The stem cells enhance the apoptosis of osteoclasts and decrease the apoptosis of osteoblasts and osteocytes. The differences in trabecular microarchitecture at various skeletal sites and site-specific variations in different diseases are determined by the heterogeneity of osteoblasts.[37]
During the primary bone formation and in states of increased bone-turnover, woven bone is produced. Trabecular bone is metabolically more active than cortical bone. The collagen fibrils are laid down alternatingly in a lamellar pattern providing strength. Bone formation is more than the resorption on the periosteal surface, whereas the reverse is true on the endosteal surface.
The bone grows both in length and radius during childhood and adolescence. The chondrocytes proliferate in the epiphyseal and metaphyseal regions resulting in longitudinal growth. Subsequently, mineralization occurs, resulting in new bone formation. Bone remodeling starts in utero and continues till death so as to maintain the strength of the bone and mineral homeostasis. This is regulated by local and systemic factors.[36,37]
Vitamin-D-Parathormone-FGF-23 axis maintains the serum calcium and phosphorus levels to facilitate bone mineralization [Figure 1]. Parathormone increases the calcium levels by bone resorption, renal reabsorption, and stimulating calcitriol synthesis. Vitamin-D helps in bone mineralization by enhancing intestinal calcium and phosphorus absorption. It stimulates osteoblast differentiation and expression of bone specific-alkaline phosphatase (ALP) and moderates the skeletal cell proliferation and apoptosis. While renal regulation is important for phosphate, intestinal control is key for calcium homeostasis.[36,37]
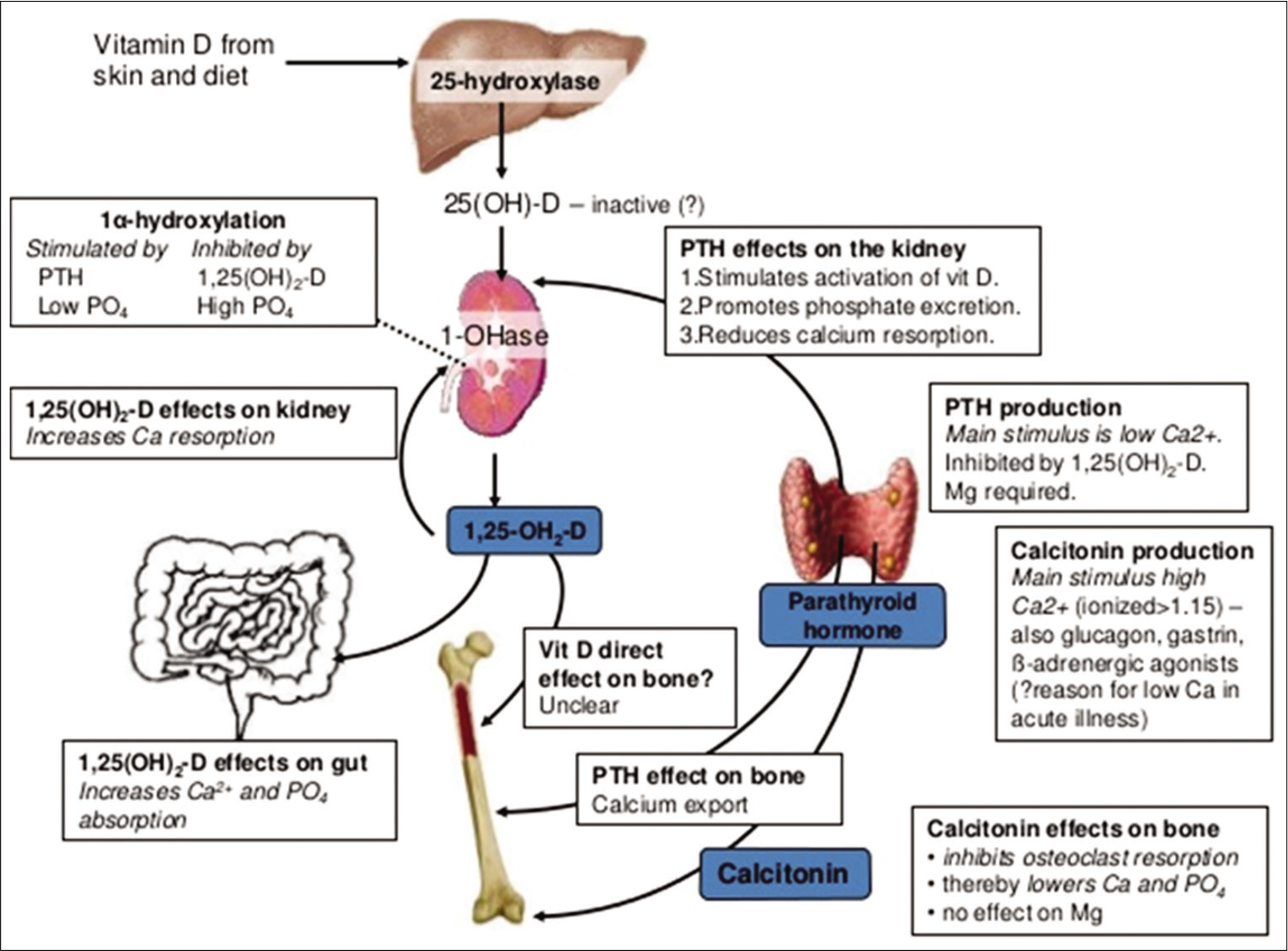
- Interplay of Vitamin-D and parathormone on calcium-phosphorus homeostasis.
ROLE OF VITAMIN-D DEFICIENCY IN ASTHMA
Awareness of low serum Vitamin-D levels in the general population is coming to light, and the reason behind this could be due to the changing lifestyle such as reduced outdoor life, working indoors, use of sunscreen, decreased sunlight exposure, and dietary modifications. Vitamin-D can contribute to lung health by reducing inflammation through regulatory T-cells and induction of intrinsic antimicrobial resistance. Hence, in asthmatic children with the lower Vitamin-D levels, it has been observed that they are more symptomatic, there is a higher risk of exacerbations, reduced pulmonary function, and increased need for reliever-use.[38] Asthmatic children seem to have a greater risk of Vitamin-D deficiency, and asthma-control in those children with Vitamin-D deficiency has been noted to be sub-optimal.[38,39]
ROLE OF STEROIDS ON VITAMIN-D METABOLISM
Glucocorticoids can reduce the serum calcium levels by impairing its absorption in the intestine, reduced calcium- binding protein synthesis, increased renal excretion, reduced reabsorption from the tubules, and depletion of mitochondrial ATP. They cause renal excretion of phosphate by directly acting on the kidney and indirectly by inducing secondary hyperparathyroidism. This hyperparathyroidism is contributed to by direct stimulation of its secretion by the steroids. Varying levels of the active form of Vitamin-D, that is, 1,25-dihydroxyvitamin-D [1,25(OH)2D] may be present. While the rate of Vitamin-D synthesis and clearance is usually normal, this variation could be due to differences in the diet, absorption, and sunlight exposure. The steroid- induced negative calcium balance can be overcome partly by supplementation of Vitamin-D and calcium.[16]
RELATIONSHIP BETWEEN VITAMIN-D AND BMD
Vitamin-D plays a significant role in bone metabolism. 1,25(OH)2D enhances bone mineralization by increasing intestinal calcium and phosphorus absorption and osteoclast maturation. In the pediatric population, the association between severe Vitamin-D deficiency and rickets and reduced BMD is well established.[40] A positive correlation was seen between Vitamin-D levels and BMD.[16]
An important function of 1,25(OH)2D is the regulation of calcium-phosphorus balance for bone mineralization and remodeling. With the deficiency of Vitamin-D, dietary calcium will not be absorbed, leading to an increase in serum parathormone, which increases tubular reabsorption of calcium and resorption from the bones at the cost of BMD [Figure 1]. In the long run, this weakens the bones and makes them brittle. About 40–60% of the skeletal mass is accumulated during childhood and adolescence. Inadequate mineralization of growing bone results in rickets, biochemical abnormalities include hypophosphatemia, elevated ALP and low serum 25(OH)D.[41]
Studies have revealed that BMD is generally low in children from India and China compared to Caucasians for corresponding height. The growth parameters were normal in Indians, but they had poorer Vitamin-D status, and their dietary calcium intake was low. Groups receiving calcium and Vitamin-D had a greater increase in bone mass than Vitamin-D alone. The appendicular bone mass increased in children with modest calcium supplementation, but the benefit was lost in 18 months after stopping the supplementation. Growth was not altered with calcium supplementation.[42] Tse et al. concluded that Vitamin-D levels significantly modified the effect of OCS on bone mineral accretion in boys. However, this study did not consider the impact of diet and physical activity on Vitamin-D levels and its link with BMD. Further research is needed to establish whether Vitamin-D supplementation in poorly-controlled asthma may confer benefits to bone health.[43]
Vitamin-D deficiency (less than 5 ng/ml) was 65% among 100 school-going children in a study by Sharawat and Dawman. In the Vitamin-D deficient group, the mean BMD (g/cm2) of lumbar-spine was 0.439 ± 0.098 and 0.606 ± 0.071 (P< 0.001) in controls.[40]
STEROID-INDUCED OSTEOPENIA
ICS is the largest form of steroid usage. Osteopenia has been noted in children even with oral prednisolone less than 0.16 mg/kg/day. OCS therapy is known to delay growth and puberty and reduce final height, temporary retardation of bone growth, and altered turnover of bone and collagen can occur even with ICS. The predominant effect on growth and BMD occurs within 6 months (pronounced effect in the first few weeks) of therapy.[44]
There are evidences demonstrating increased fracture risk with chronic OCS, but such strong evidence is not found with chronic ICS. A study of bone biopsy among patients on steroid therapy for at least 1 year showed an increased resorption, reduced formation, and reduced volume of trabecular bone, the loss being higher in metaphysis than diaphysis. Although the trabecular bone is mostly affected, with prolonged therapy, the cortical bone also becomes affected, increasing the fragility of long bones. Most asthmatics are on low-dose steroids, but some show an increased susceptibility to steroid- induced adverse effects on the bone than others, implying the role of genetic differences along with other factors such as dose and duration of therapy.[45]
The specialty of corticosteroid-induced osteoporosis is that there is rapid initial bone loss. There is a dual disadvantage as there is both decreased bone formation and increased resorption, leading to increased fractures risk at a higher BMD. This risk reduces on stopping the steroids, but, the bone recovery is not complete. The specific sites involved in this condition include the cancellous bone such as the spine, neck of femur, and the Ward’s triangle.[45]
Along with the steroid, the underlying disorder itself can affect the bone health independently by local cytokine- induced bone resorption, malabsorption states, reduced physical activity, hypoxia, and acidosis. Other factors which add to the burden of osteoporosis include age, gender, and genetic influence, endocrine disorders such as hyperthyroidism, hyperparathyroidism, Cushing’s disease, gonadal-insufficiency, and Type-1 diabetes mellitus, and other diseases such as chronic kidney disease, inflammatory bowel disease, and rheumatoid arthritis. In the pediatric age- group, predisposing factors to low-BMD include overweight/ obesity, reduced muscle mass, and low physical activity.[45] The review article by Seibel et al. in 2013 summarized that the therapeutic use of glucocorticoids is often limited by adverse outcomes such as osteoporosis, diabetes, and obesity.[12]
The route of steroid administration plays an important role with respect to the equivalent dose and the corresponding effect on the BMD. While a high-dose of up to 1 g methylprednisolone given intravenously may not be taxing to the bone, prednisone, when given as a 2.5 mg single oral dose, can have an immediate detrimental effect on the secretion of osteocalcin. With regard to bone health, though ICS does have an impact on the skeletal tissue, they are better than systemic or OCS.[44,45]
Steroids can affect bone health through a multitude of mechanisms [Figure 2].[46] They suppress genesis of osteoblasts, decrease bone formation by promoting the apoptosis of osteocytes and osteoblasts, extend the lifespan of osteoclasts thus enhancing bone resorption, promote a renal and intestinal calcium loss, impair bone growth through direct effect on the physis, and delay/impair the attainment of peak bone mass.[44]
![Effects of glucocorticoids on bone.[46]](/content/113/2020/35/1/img/KPJ-35-039-g002.png)
- Effects of glucocorticoids on bone.[46]
ICS AND BMD
Local and systemic side effects of ICS are a major concern on a long-term use. Parameters influencing the side effects: Local drug deposition, type of drug, frequency, and dose.[47] A higher dose can lead to suppression of hypothalamic-pituitary-adrenal axis.[48] Common systemic side effects include lower respiratory infection, tuberculosis, growth-suppression, reduced BMD, ocular side effects, cutaneous infections, and easy bruisability.[49,50]
In children on ICS, there can be a growth retardation. The impact of steroids on bone metabolism is based on the route of administration, dose and bioavailability of steroids. Intranasal steroids can lead to systemic absorption and reduction in growth velocity. Although studies in asthmatic children have not shown a significant reduction in BMD, an error may be that children on low-dose ICS were taken into consideration.[44]
Fuhlbrigge and Kelly [Table 1], in their review, concluded that long-term ICS had a greater impact on growth, while BMD is more sensitive to short-courses of OCS.[11] Another review article by Mortimer et al., with databases from 1966 to 2004, concluded that with ICS therapy, though there was a reduction in growth velocity, target adult height was achieved.[13] When metered-dose ICS is used with spacers, compliance to therapy and efficacy is improved, local and systemic side effects such as oral candidiasis, dysphonia, suppression of growth, and loss of BMD can be reduced.[51] In the case of dry powder inhalers, rinsing the mouth after inhalation reduces the systemic absorption of steroids.[52]
| Study | Steroid | Duration | Age-years | Sample size | Outcome |
|---|---|---|---|---|---|
| Jones et al.[16](2000-Tasmania) | ICS 479 μg/day (range 50–1000) | 8 years follow-up | 8.2 (median) | 330 | |
| (110 asthma-51 used ICS) | ICS use in the past 1 year was associated with decreased total-body-BMD, for doses >400 mcg/day | ||||
| Harris et al.[14](2001-Australia) | Moderate-dose ICS (400–800 μg), high-dose ICS (800 μg), high-dose ICS+OCS, no ICS | 6 months of ICS | 4–12 | 76 | Children on >800 μg/day ICS + intermittent OCS had a significantly lower Lumbar-Spine BMD than 400–800 μg/day of ICS |
| Bone mass was similar in children not on ICS and those on 400–800 μg/day ICS | |||||
| Van Staa et al.[17](2003-UK) | OCS versus non-systemic corticosteroids | Treatment duration: 6.4 days | 4–17 | 37562 cases, 345748 controls | Children who need >4 OCS courses had a dose-dependent increased risk offracture humerus. Risk was increased with ≥30 mg prednisolone/day |
| Tse et al.[43](2012-Boston) | Inhaled budesonide 400 μg), nedocromil (16 mg), or placebo | Mean 4.3 years follow-up | 5–12 | 780 | Vitamin-D levels significantly modified the effect of OCS on bone mineral accretion in boys. Vitamin-D-insufficient boys exposed to >2 OCS courses/year had twice the decrease in accretion rate |
| Sidoroff et al.[18](2015-USA) | ICS: Budesonide 1000 μg for 8 weeks, 500 μg for 8 weeks | >6 months | 12.3 (median) | 89 | Regular use of ICS <6 years of age was associated with reduced lumbar spine-BMD later in childhood |
| High cumulative ICS-dose was associated with decreased BMD in the femoral neck | |||||
| Bahceciler et al.[15](2002-Turkey) | Long-term inhaled budesonide (mean daily dosage: 419±154 μg) | 13.0±9.8 months | 6.4±2.2 | ||
| (22 males, 30 females) | 52 cases, 22 controls | No significant difference in total and spine BMD | |||
| Griffiths et al.[19](2004-Australia) | High-dose inhaled fluticasone propionate (≥1000 μg daily) | 6 months | 13.63±3.2 | 49 cases, 32 controls | No significant reduction in bone metabolism or bone-age corrected BMD |
| Zieck et al.[20](2017-Australia) | Asthmatics on ICS versus non-asthmatics not on ICS | 6 months | 6–18 | 211 cases, 216 controls | No difference in the incidence of fractures with low or high-dose ICS |
| Kelly et al.[53](2008) | OCS (2 mg/kg for 2 days, 1 mg/kg for 2 days) versus ICS (400 μg/day budesonide) | Median 7 years follow-up | 5–12 | 531 boys, 346 girls | OCS bursts produced a dosage-dependent reduction in bone mineral accretion and an increase in risk for osteopenia in boys. Cumulative ICS usewas associated with a small decrease in bone mineral accretion in boys but no increased risk for osteopenia |
| Allen et al.[54](2000-Australia) | Inhaled beclomethasone dipropionate or budesonide (0.67±0.48 mg/m2/day) | 9–20 month follow-up | Cases: 7.8±2.4 controls: 8.4±2.1 | 48 cases, 9 controls | ICS at an average dose of |
| 0.67 mg/m2/day reduced the bone mineral acquisition | |||||
| Boot et al.[55](1997-Netherland) | Moderate-to-high dose ICS. Beclomethasone dipropionate or budesonide | 6 months-2 years | 7 (median) | 40 (21 boys, 19 girls), 148 controls | Lumbar spine-BMD was not affected byICS. Children who used ICS daily for 3–8 years had lower total-body-BMD |
MANAGEMENT OF CORTICOSTEROID- INDUCED OSTEOPENIA
Chronic steroid therapy can lead to reduction in bone mineral density.[53-55] So, it is important to have a watchful eye. The early changes in BMD can be detected by dual-energy X-ray absorptiometry and quantitative computed-tomography. Choi et al., in 2019, used an indirect parameter-trabecular bone score, calculated using projections of DEXA in the lumbar spine, and found it useful as an early indicator of bone loss.[56] However, this was a retrospective study, and it included subjects on a short duration of controllers. It is preferable to do a biannual assessment in those not on preventive therapies and annually in those who are receiving preventive therapy. We should use the lowest-effective-dose of steroids for chronic therapy.[45]
The goal is to minimize or prevent further bone loss, enhances the BMD, and try to revert the effects of glucocorticoid excess.[45] Studies have revealed that bisphosphonates, which are anti-resorptive agents, help prevent bone-loss or improve BMD. Among this class of drugs, only pamidronate and alendronate were studied. While reducing the osteoclastic resorptive activity, they simultaneously enhance the apoptosis of osteoclasts, and reduce the apoptosis of osteocytes and osteoblasts. Hence, they are beneficial for the prevention and treatment of glucocorticoid-induced osteoporosis.[45]
A combination of Vitamin-K2 (menatetrenone) with alfacalcidol helps in preserving BMD. Calcium-alfacalcidol combination is more effective in preventing bone loss than Vitamin-K2. Calcium-calcitriol combination reduces the bone loss, but cannot prevent it altogether. It is better to start a combination of Vitamin-D with calcium rather than calcium alone with any chronic therapy. Children on long-term glucocorticoid therapy or with reduced BMD should be considered for treatment with bisphosphonates along with calcium and Vitamin-D supplementation.[49] Calcium and protein-rich low-sodium diet, good physical activity, and protection from falls help prevent reduction in BMD.[45]
Other modalities of treatment include anabolic medications, recombinant parathormone, and sex hormones. Newer therapeutic agents in the pipeline are recombinant- osteoprotegrin, receptor activator of nuclear-factor-kB ligand inhibitors, osteoclast enzyme inhibitors, and antagonists of integrin.[45] Sirufo et al., in 2020, found that nuclear-factor- kB has a key role in allergy-induced inflammation and regulation of bone resorption, hence a potential target for therapy and further research. A definite link exists between bone-loss, therapies, and etiology, but the final effect is based on mutual interaction of various factors.[57]
CONCLUSION
ICS is an integral part of asthma management. However, chronic high-dose therapy can cause a reduction in BMD and pose a risk for fractures. There are conflicting evidences in the literature regarding the impact of ICS on BMD. Benefits of therapy outweigh the risk of adverse effects. However, it is imperative to use steroids judiciously with appropriate monitoring and preventive measures in anticipation of the side effects.
Recommendations to prevent the risk of ICS-induced osteopenia
Use the lowest-effective dosage of ICS
Annual assessment of BMD for children who have received at least 6 months of ICS therapy
Start Vitamin-D and calcium supplementation for children predicted to need chronic therapy
Treat Vitamin-D insufficiency and deficiency and continue monitoring Vitamin-D status
Use metered-dose inhalers with spacers and stress on good compliance to avoid the need of burst OCS courses
While using inhaler, rinse the mouth after each use
Advise appropriate non-pharmacological methods to improve bone health such as exercise, calcium, and protein-rich diet.
Declaration of patient consent
Patient’s consent not required as patients identitiy is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Global Strategy for Asthma Management and Prevention. 2020. Available from: https://www.ginasthma.org [Last accessed on 2020 Apr 24]
- [Google Scholar]
- The Global Asthma Report. 2018. Auckland, New Zealand: Global Asthma Network; Available from: http://www.globalasthmareport.org [Last accessed on 2018 Oct 17]
- [Google Scholar]
- Nation Current Asthma Prevalence. 2014. Atlanta, Georgia, United States: Centre for Disease Control and Prevention; Available from: http://www.cdc.gov/asthma/nhis2014/table3-1 [Last accessed on 2018 Oct 17]
- [Google Scholar]
- Worldwide variation in prevalence of asthma allergic rhinoconjunctivitis and atopic eczema: ISAAC. The international study of asthma and allergies in childhood (ISAAC) steering committee. Lancet. 1998;351:1225-32.
- [CrossRef] [Google Scholar]
- Prevalence of asthma in urban and rural children in Tamil Nadu. Natl Med J India. 2002;15:260-3.
- [Google Scholar]
- Prevalence of asthma symptoms in 7th-and 8th-grade school children in a Rural Region in India. J Asthma. 2008;45:117-22.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of bronchial asthma in rural Indian children: A cross sectional study from South India. Indian J Pediatr. 2010;77:31-5.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of corticosteroids on growth and bone health. Arch Dis Child. 2002;87:93-6.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma training module (ATM), asthma by consensus (ABC) and asthma education. Indian Pediatr. 2011;48:433-5.
- [CrossRef] [PubMed] [Google Scholar]
- Inhaled corticosteroids in children: Effects on bone mineral density and growth. Lancet Respir Med. 2014;2:487-96.
- [CrossRef] [Google Scholar]
- Glucocorticoid-induced osteoporosis: Mechanisms, management and future perspectives. Lancet Diabetes Endocrinol. 2013;1:59-70.
- [CrossRef] [Google Scholar]
- Effects of inhaled corticosteroids on bone. Ann Allergy Asthma Immunol. 2005;94:15-21.
- [CrossRef] [Google Scholar]
- Bone mineral density in prepubertal asthmatics receiving corticosteroid treatment. J Paediatr Child Health. 2001;37:67-71.
- [CrossRef] [PubMed] [Google Scholar]
- Inhaled corticosteroids and bone density of children with asthma. J Asthma. 2002;39:151-7.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma, inhaled corticosteroid use, and bone mass in prepubertal children. J Asthma. 2000;37:603-11.
- [CrossRef] [PubMed] [Google Scholar]
- Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res. 2003;18:913-18.
- [CrossRef] [PubMed] [Google Scholar]
- Inhaled corticosteroids and bone mineral density at school age: A follow-up study after early childhood wheezing. Pediatr Pulmonol. 2015;50:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of high-dose fluticasone propionate on bone density and metabolism in children with asthma. Pediatr Pulmonol. 2004;37:116-21.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma, bones and corticosteroids: Are inhaled corticosteroids associated with fractures in children with asthma? J Paediatr Child Health. 2017;53:771-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of inhaled corticosteroids on bone density in patients with asthma. J Allergy Clin Immunol Suppl. 2018;141:AB212.
- [CrossRef] [Google Scholar]
- Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054-63.
- [CrossRef] [PubMed] [Google Scholar]
- Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179:19-24.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the management of work-related asthma. Eur Respir J. 2012;39:529-45.
- [CrossRef] [PubMed] [Google Scholar]
- Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database Syst Rev. 2001;1:CD001740.
- [CrossRef] [Google Scholar]
- Need for intravenous hydrocortisone in addition to oral prednisolone in patients admitted to hospital with severe asthma without ventilatory failure. Lancet. 1986;1:181-4.
- [CrossRef] [Google Scholar]
- Systemic steroids for the treatment of acute asthma: Where do we stand? Clin Pulm Med. 2006;13:315-20.
- [CrossRef] [Google Scholar]
- Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;1:CD002178.
- [CrossRef] [Google Scholar]
- Duration of systemic corticosteroids in the treatment of asthma exacerbation; a randomized study. Intern Med. 2000;39:794-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective, placebo controlled trial of 5 vs 10 days of oral prednisolone in acute adult asthma. Respir Med. 2002;96:950-4.
- [CrossRef] [PubMed] [Google Scholar]
- Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD002308.
- [CrossRef] [Google Scholar]
- Guidelines for diagnosis and management of bronchial asthma: Joint ICS/NCCP (I) recommendations. Lung India. 2015;32:3-42.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of inhaled fluticasone with intravenous hydrocortisone in the treatment of adult acute asthma. Am J Respir Crit Care Med. 2005;171:1231-6.
- [CrossRef] [PubMed] [Google Scholar]
- Inhaled steroids for acute asthma following emergency department discharge. Cochrane Database Syst Rev. 2012;12:CD002316.
- [CrossRef] [Google Scholar]
- Bone structure growth and hormonal regulation. Nelson Textbook of Paediatrics. 2020;1:3746-7.
- [Google Scholar]
- Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3:S131-9.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D and asthma in children. Paediatr Respir Rev. 2012;13:236-43.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D Deficiency as a factor influencing asthma control in children. Indian Pediatr. 2018;55:969-71.
- [CrossRef] [PubMed] [Google Scholar]
- Bone mineral density and its correlation with Vitamin D status in healthy school-going children of Western India. Arch Osteoporos. 2019;14:13.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D deficiency in India: Prevalence, causalities and interventions. Nutrients. 2014;6:729-75.
- [CrossRef] [PubMed] [Google Scholar]
- Calcium and Vitamin D metabolism in children in developing countries. Ann Nutr Metab. 2014;64(Suppl 2):15-22.
- [CrossRef] [PubMed] [Google Scholar]
- Corticosteroid use and bone mineral accretion in children with asthma: Effect modification by Vitamin D. J Allergy Clin Immunol. 2012;130:53-60.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of corticosteroids on growth and bone health. Arch Dis Child. 2002;87:93-6.
- [CrossRef] [PubMed] [Google Scholar]
- An overview of glucocorticoid induced osteoporosis. Endotext, South Dartmouth, MA: MDText.com, Inc.; 2000.
- [Google Scholar]
- The risk of osteoporosis in patients with asthma. Eur Clin Respir J. 2020;7:1763612.
- [CrossRef] [PubMed] [Google Scholar]
- The local side effects of inhaled corticosteroids: Current understanding and review of the literature. Chest. 2004;126:213-9.
- [CrossRef] [PubMed] [Google Scholar]
- Ciclesonide: A closer look at its systemic and oropharyngeal safety profile. Curr Drug Saf. 2006;1:265-70.
- [CrossRef] [PubMed] [Google Scholar]
- Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child. 2002;87:457-61.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100:1307-17.
- [CrossRef] [PubMed] [Google Scholar]
- Hold it! Correct use of inhalers in children with asthma. West J Med. 2001;175:303-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of a volumatic spacer and mouth rinsing on systemic absorption of inhaled corticosteroids from a metered dose inhaler and dry powder inhaler. Thorax. 1991;46:891-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of long-term corticosteroid use on bone mineral density in children: A prospective longitudinal assessment in the childhood Asthma Management Program (CAMP) study. Pediatrics. 2008;122:e53-61.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of high-dose inhaled corticosteroids on bone metabolism in prepubertal children with asthma. Pediatr Pulmonol. 2000;29:188-93.
- [CrossRef] [Google Scholar]
- Bone mineral density and bone metabolism of prepubertal children with asthma after long-term treatment with inhaled corticosteroids. Pediatr Pulmonol. 1997;24:379-84.
- [CrossRef] [Google Scholar]
- Trabecular bone score is more sensitive to asthma severity and glucocorticoid treatment than bone mineral density in asthmatics. Allergy Asthma Immunol Res. 2019;11:343-56.
- [CrossRef] [PubMed] [Google Scholar]
- Does allergy break bones? Osteoporosis and its connection to allergy. Int J Mol Sci. 2020;21:712.
- [CrossRef] [PubMed] [Google Scholar]






