Translate this page into:
Magnetic resonance imaging brain sequences in pediatrics
*Corresponding author: Ravindra Bhimrao Kamble, Department of Radiology, Aster RV Hospital, Bengaluru, Karnataka, India. drravindra31@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kamble RB. Magnetic resonance imaging brain sequences in pediatrics. Karnataka Paediatr J 2021;36(1):27-34.
Abstract
There are various pediatric magnetic resonance imaging (MRI) protocols followed in institutes and by individual radiologists, determined by the disease process and the indication for imaging, to narrow down the differential diagnosis. Most times, it is beneficial to modify protocols when the scans are being done, based on the findings seen on initially acquired sequences. This is particularly useful in pediatric patients considering most of them are scanned either under sedation or general anesthesia, and repeat scans will be cumbersome. In this particular review article, we are going to discuss appropriate MRI sequences in scanning pediatric brains and the need for rapid MRI sequences. This is of immense importance as MRI in pediatric patients poses challenges both to radiologists and technologists. Consequently, appropriate MRI protocols should be set to avoid repeat studies.
Keywords
Magnetic resonance imaging
Protocols
Rapid sequences
Pediatric
Brain
INTRODUCTION
Magnetic resonance imaging (MRI) brain sequences in pediatrics should be standardized in such a way that the scans are done in minimum time, have great image quality without artefacts, and sufficient enough to arrive at the diagnosis. The use of advanced imaging techniques such as perfusion, tensor imaging, or spectroscopy is rarely required. Although most of the protocols can be designed as per the clinical indication and age of the patient, scans should be monitored by the radiologist, and the need to modify sequences or use IV contrast be justified. The technologists should be trained to apply particular protocols in children and be confident enough to modify even when the radiologist is not around. The technologists must be well aware of rapid MRI protocols which are particularly useful in patients less than 2 years of age.
CHALLENGES ENCOUNTERED IN SCANNING PEDIATRIC BRAINS
The major challenge is to keep the children still through the scan duration. This can be done either by intravenous sedation, general anesthesia, or oral sedation. Older children usually are more co-operative, but the younger ones require some sort of intervention. Apart from sedation, premature infants pose further challenges as there is a need for maintaining body temperature and safe transport. A trained nurse or neonatologist should accompany the neonate to monitor the homeostasis during the scans. Even MR compatible incubators can be used for transport and during the scan without degrading the image quality.[1] Not so sick infants usually sleep after feed and rarely require sedation. Sedation should be given by a trained nurse, who should also be responsible for the child till recovery and help in obtaining good image quality without motion artefact.[2] Most institutes or hospitals, including ours, prefer anesthetists or pediatricians to administer sedation or general anesthesia. All sedated infants and children should be monitored for cardiorespiratory parameters throughout the scan until recovery. Furthermore, care should be taken to keep the children warm and use earplugs or earmuffs to avoid movement.[3] Older children between 2 and 5 years can be given oral sedatives such as chloral hydrate or Phenergan for completing the scans. Sometimes, intravenous sedation like propofol with an infusion dose of 2–5 mg/kg/h can be used with short induction and early recovery. Other intravenous drugs which are used for sedation are dexmedetomidine (loading dose 2–3 μg/kg and maintenance infusion 1–2 μg/kg) and pentobarbital.[4-6]
Almost all institutes or hospitals have adult MRI coils, while very few pediatric institutes have dedicated pediatric coils, which have an obvious advantage in terms of image quality compared to adult coils. However, it is always preferred to use high filed magnets like 3 Tesla MRI with dedicated multichannel phased array coil, fast imaging sequences, parallel imaging, compressed sensing to reduce scan time and improve the image quality.[7,8]
Whatever coils used, pediatric brain imaging should be done with a slice thickness of 5 mm for brain and 3 mm for orbit and pituitary scans. Some authors prefer to use advanced multichannel head and spine coils for pediatric imaging and avoid ultrafast breath-hold T2W imaging with low signal to noise ratio for obvious reasons.[9]
USE OF CONTRAST IN PEDIATRIC BRAIN IMAGING
As in adults, all precautions should be taken before the administration of intravenous MRI contrast for safety purposes. Prior renal function tests should be done. In the majority of the cases, contrast is seldom required. The common indications for contrast in pediatric brains include infection, demyelination, tumors, metastases, CSF and cranial nerve pathologies, neurocutaneous syndromes, and vascular pathologies. Sometimes, contrast is used to perform specific functional scans like perfusion-weighted imaging or contrast-enhanced angiography, depending on the clinical need. The advantages of contrast imaging include detecting, localizing, and characterizing the lesions and in follow-up imaging, especially post-treatment tumors and demyelinating diseases.[10]
Various gadolinium-based MRI contrast is available such as gadodiamide, gadopentetate dimeglumine, and gadobenate dimeglumine which have a linear polyaminocarboxylic acid structure that incompletely encircles gadolinium ions, whereas macrocyclic agents such as gadobutrol, gadoterate meglumine, and gadoteridol completely encircle the gadolinium ions thus making them more stable and safe especially in terms of developing nephrogenic systemic fibrosis.[11-14]
Dosage is weight dependent just as in adults (0.1 mmol/kg body weight) and is not based on age. This is proved even in pediatric patients below 2 years with gadobutrol.[15] Most of the contrast agents are in 0.5 molar concentration except gadobutrol which has 1 molar concentration. In general, gadobutrol is considered a safe and efficient MRI contrast agent in children.[10]
STANDARD MRI BRAIN SEQUENCES
MRI has specific advantages over other modalities such as computed tomography – no ionizing radiation, increased tissue contrast, multiplanar multisequence capabilities, and functional imaging. Combinations of various sequences help in diagnosis and the aim is to apply the most appropriate sequence to reduce time and acquire good quality images.
SEQUENCES FOR ASSESSMENT OF MYELINATION
Pediatric brain undergoes rapid changes in the first 2 years in terms of growing myelination and reducing brain water content. Hence, it is important that proper MRI sequences are selected to differentiate pathology from normal myelination and also to look for the occurrence of age-appropriate myelination which requires good tissue contrast.[16]
Up to the first 6 months of life, T1-weighted images are useful, which show hyperintensity of myelinated fibers (dark to bright); thereafter, up until 2 years, T2-weighted images are useful where the maturing myelinated fibers appear hypointense (bright to dark). Due to poor gray matter white matter differentiation before 6 months of age, subtle subcortical lesions can be obscured, but after myelination which appears T1W bright, the subcortical pathologies can be seen on T1W images.[17] Fluid attenuation inversion recovery (FLAIR) is not particularly useful in children due to high water content.[9] Typically, FLAIR sequence shows a triphasic pattern in children. In young infants, deep cerebral white matter is heterogeneously hypointense (relative to gray matter), in early months, it becomes hyperintense and then in 2nd year of life, it again becomes hypointense. This pattern must be known to avoid misinterpretations.[18]
Similarly, due to unmyelinated fibers and high water content, diffusion-weighted images are also not very useful in the early period of life. It shows a significant reduction in ADC with advancing age.[19]
Dual-echo short-tau inversion recovery (STIR) sequence is also useful below 2 years which shows increased contrast resolution (TE 30/128 ms, TR 5,400 ms, and TI 130 ms).[9,20]
Instead of T1W sequence, 3D gradient-echo T1-weighted pulse sequence like magnetization prepared rapid gradient echo (MP-RAGE) can be used.
In summary, to detect age-appropriate myelination, spin-echo (SE) T1W or 3D MP-RAGE, T2W, and STIR axial sequences can be used.
ROUTINE BRAIN SEQUENCES
In some dedicated pediatric institutes, axial T2W fast spin-echo (FSE) and coronal FLAIR, sagittal and coronal T1W SE, DWI, and T2* gradient sequence like susceptibility-weighted imaging (SWI) is used in children more than 2 years. In addition, in children below 2 years, dual-echo STIR axial and coronal is used instead of T2W FSE. SWI sequence specifically is added to assess any blood products. If contrast is given, then coronal/axial SE T1W with magnetization transfer (MT) are added. In neonates, slice thickness can be reduced to 4 mm, matrix size and FOV can be reduced, and adult knee coil can be used to improve the image quality.[9]
In our practice, we follow 3D FLAIR, 3D MP-RAGE, DWI/ T2W/SWI axial, and T2W coronal sequences. Inclusion of these help assesses midline structures like corpus callosum and brainstem and anterior structures like optic nerves and optic pathway, para cavernous, and orbital/paranasal sinuses. 3D sequences are more useful to assess periventricular structures and anterior-most middle cranial fossa lesions. If contrast is given, then post-contrast 3D T1 MP-RAGE and axial T1W with MT are used.
BRAIN SEQUENCES IN VARIOUS DISEASES
Orbit, inner ear, and pituitary lesions
To assess orbital lesions, including optic nerves and optic pathway and the pituitary, dual-echo STIR and T1W SE fat-saturated (FS) sequence in coronal and axial planes are used to suppress orbital fat with a slice thickness of 3 mm and small matrix size to increase tissue contrast. If contrast is given, then FS T1W axial and coronal is used. For pituitary lesions, sagittal and coronal T1W and coronal T2W with additional T1W coronal and sagittal post-contrast are used. For inner ear structures, including internal auditory canal, heavily T2W sequences such as CISS 3D (SIEMENS), FIESTA in GE, and DRIVE in Philips are used apart from the standard.[9]
We use thin 3 mm slice thickness, axial and coronal STIR or FS T2W, and sometimes oblique sagittal in plane with optic nerves to assess orbit and optic nerves. If contrast is used, then FS T1W axial and coronal are added. Same sequences can be used to assess paranasal sinuses and the pituitary gland with the addition of FS T1W sagittal. Dynamic contrast study in coronal planes for the pituitary gland can be used if indicated. Post-contrast T1 MP-RAGE has not been found useful to assess contrast enhancement in the pituitary gland or orbit due to air (of paranasal sinuses) tissue interface artefacts.
Brain tumors
For characterization of brain tumors in terms of location, size, multiplicity, spread, and complication like hydrocephalus, standard brain sequences with contrast as described above are used. In addition, all children with brain tumors should have their spine imaged, preferably with contrast T1W FS sequence, to detect distant metastases.[21] It is wise to use a volumetric 3D sequence such as pre-contrast FLAIR or post-contrast T1W (MP-RAGE) without using ear muffs for neuronavigation guided surgery.
Advanced imaging techniques such as MR contrast enhanced dynamic perfusion (DSC), arterial spin labeling perfusion (ASL), MR spectroscopy, and diffusion tensor imaging are usually used to characterize tumor type but rarely required in children. However, these advanced imaging techniques are useful in post-operative children who have received radiation to differentiate between tumor recurrence and radiation necrosis. In children, ASL perfusion study can be useful which does not require contrast.[22]
Immediate post-operative MRI scans within 72 h are usually performed with contrast to avoid interference of postoperative changes such as edema and blood products with tumor residue.[21] Sometimes intra-operative MRI scans are done to assess tumor residue, preferably with contrast. It is important to find pre-operative image characteristics of tumors while doing intra-operative scans to use appropriate sequences which can highlight the residual tumor.
Follow-up MRI brain scans in treatment received brain tumors should include imaging of the spine even if there is no residual tumor since early detection of spinal metastases helps improve overall survival.[23,24]
We prefer to add post-contrast FLAIR axial for brain and FS T1W sagittal sequence for spinal imaging to detect distant metastases. Post-contrast FLAIR is found to be more useful to detect leptomeningeal metastases.[25]
Infections
Standard imaging protocols should be used along with contrast to characterize the type of lesions. Usually, in meningitis and meningoencephalitis, addition of post-contrast FLAIR along with T1W MT sequence yields better visualization of meningeal enhancement.[26] In our practice, both post-contrast FLAIR and T1W with MT are found useful; however, 3D T1 MP RAGE sequence is not found to be adequate to evaluate meningeal enhancement [Figure 1]. Sometimes it is useful to find the source of infection such as mastoiditis or sinusitis and add additional sequences accordingly. Screening of spine with post-contrast T1W sagittal FS sequence will show any changes of spinal meningitis.
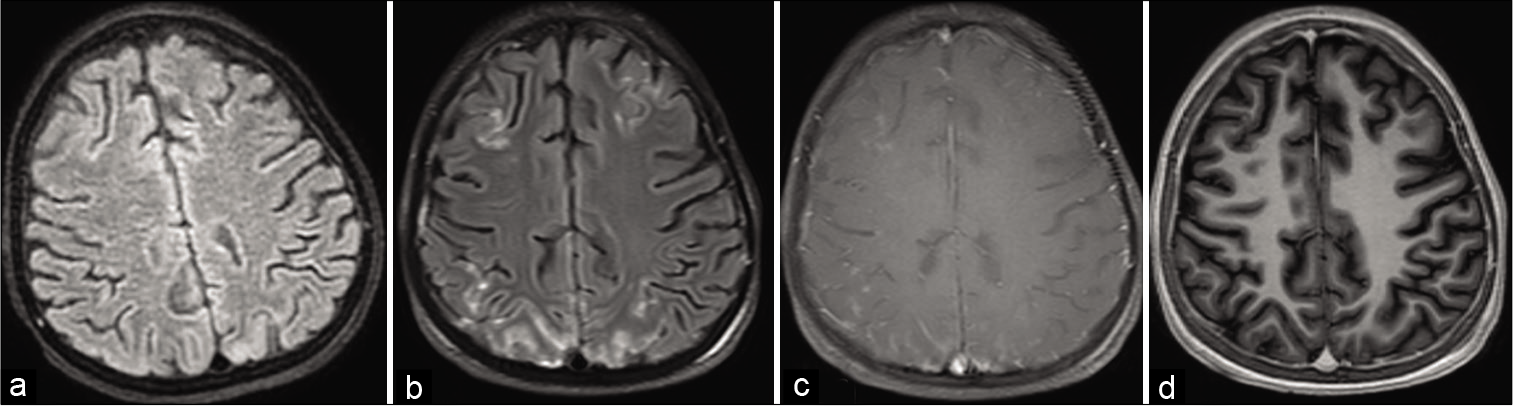
- (a) Non-contrast fluid attenuation inversion recovery (FLAIR), (b) post-contrast FLAIR, (c) post-contrast T1W with magnetization transfer (MT), (d) axial reconstruction of 3D T1 magnetization prepared rapid gradient echo (MP-RAGE). In this case of meningitis, meningeal enhancement is clearly visible on post-contrast FLAIR and T1 with MT and not seen in T1 MP-RAGE.
In cases of ring-enhancing lesions, the addition of heavily T2W sequence, DWI, SWI, MR spectroscopy, or perfusion will help to accurately arrive at the diagnosis. These advanced imaging sequences will help differentiate between metastatic ring lesion, abscess, tuberculoma, or neurocysticercosis. CISS sequence is useful to detect scolex in cysticercosis, SWI is useful to detect calcification in granulomatous disease and detect hemorrhage in hemorrhagic meningoencephalitis, DWI is useful to detect pyogenic abscess and spectroscopy and perfusion to differentiate neoplastic from non-neoplastic lesions.[27-30]
Hydrocephalus
Hydrocephalus can be communicating or obstructive. In addition to routine imaging protocol, 3D T2W SPACE/ heavily T2W sequences such as CISS 3D and SWI should be added. 3D T2W SPACE and heavily T2W sequences help identify the type. SWI sequence helps to identify any blood products or previous hemorrhage as a cause of communicating hydrocephalus.[31] Contrast MRI can be useful to detect the infective cause of communicating hydrocephalus.
CSF flow study can add value in detecting the cause of hydrocephalus. It can be done across the aqueduct or foramen magnum in case of Chiari malformation. It is also useful to detect flow patterns in intracranial arachnoid cysts and in shunt malfunction.[32]
Metabolic disease and leukodystrophy
In evaluating children with leukodystrophy, majority of the time, standard brain protocol is sufficient. In addition, spine screening with T2W sagittal can be done to assess spinal cord involvement and MR spectroscopy to detect metabolite abnormalities. MR spectroscopy can show pathognomonic findings in a few leukodystrophies like Canavan disease and metabolic diseases like mitochondrial cytopathies.[33]
Demyelinating diseases
In addition to routine standard imaging protocols, imaging of orbits and spinal screening should be performed, preferably with contrast. Orbital coronal STIR is used to detect optic neuritis and enhancement on coronal FS T1W. Spinal screening can be done with T2W sagittal and post-contrast T1W FS sagittal to detect spinal lesions and their enhancement. Post-contrast FLAIR will be useful especially to detect periventricular lesions and their enhancement. 3D FLAIR or sagittal FLAIR adds value to detect corpus callosal lesions. Similar protocols should be followed in follow-up scans for studying the comparative images.[34]
Epilepsy
Imaging protocols in children with epilepsy differ from routine imaging protocol. A few centers use axial T2W fast spin-echo, 3D T1W volume acquisition reconstructed in three planes, coronal T2W fast spin-echo, coronal FLAIR (or 3D FLAIR), and hippocampal T2-relaxometry. In children less than 2 years, coronal T2W is replaced by dual-echo STIR sequence. The coronal sequences are used with planes perpendicular to hippocampus.[9] T2 relaxometry is rarely used today, but it is useful to obtain T2 values in cases of mesial temporal sclerosis, especially if it is bilateral.[35]
In our institute, we use 3D FLAIR, 3D MP-RAGE T1W, 3D TSE single slab SPACE, DWI axial, FLAIR axial parallel to hippocampus, coronal dual-echo STIR or T1W IR, and T2W coronal perpendicular to hippocampus with 3 mm slice thickness and SWI. With the use of these sequences, most of the migrational anomalies, temporal and extratemporal lesions, and calcifications/hemorrhagic lesions can be detected. We recommend use of 3D T2W SPACE sequence which is a single slab 3D T2W image (PHILIPS) with the ability to reconstruct in all three planes without degrading image quality.
In some children with refractory epilepsy and normal MRI in the first 2 years of life, a second scan after brain maturation (at least 6 months apart) to detect the focus of epilepsy is required.[36]
The use of 3 tesla MRI is more useful in detecting the focal epileptogenic lesions as compared to 1.5T MRI due to better image uniformity, signal to noise ratio, and spatial resolution.[37,38]
Functional MRI is specifically useful in the presurgical evaluation of the sensorimotor cortex or language lateralization. Ictal/interictal PET or SPECT are additional modalities to detect seizure focus apart from MR spectroscopy, magnetoencephalography, MT imaging, and diffusion tensor imaging.[39]
Children presenting with non-traumatic brain hemorrhage
Apart from standard imaging protocol, 3D time of flight (TOF) MR angiography, 2D or 3D phase-contrast MR venogram, and contrast can be used in non-traumatic brain hemorrhage. Sometimes if the child is not stable, CT angiography can be performed. MRI with MR angiogram or venogram should be performed after 72 h of ictus to avoid signal interference from hemorrhage. Contrast sequences are useful to detect any underlying tumor or vascular malformation such as cavernoma or small arteriovenous malformation.[9,40] We additionally add SWI sequence to detect any previous petechial hemorrhages, which could support the diagnosis of vasculitis or multiple cavernomas.
FAST/ultrafast MRI sequences can be used in unstable patients such as FAST FLAIR, EPI FLAIR, EPI DWI, single-shot FSE T2W/T1W by use of parallel imaging technique or susceptibility-weighted angiography, and HASTE sequence in coronal or axial planes, which reduce the scan time significantly and avoid the need for CT scan.[41-43]
Children presenting with stroke
The most important causes of stroke in children are cardiac, hematologic, oncologic, infective, vasculopathy/vasculitis, trauma, or drug-induced vasculopathy.[44] The imaging protocols should be set to detect any of these causes. 3D TOF MR angiography along with DWI and SWI should be done. In 80% of stroke cases, abnormalities are found in the intracerebral arteries, so doing MR angiography is very important.[45] Extracranial MR angiography with axial dual-echo STIR and FAT SAT T1W sequences of the neck will help detect arterial dissection of neck vessels and also identify vascular wall hemorrhage, especially in case of trauma.[46] Rapid and ultrafast MRI sequences can be additionally used to fast screen the suspected stroke patients where stroke mimics can be identified by FLAIR sequence.[47]
Children with non-accidental brain trauma
Although CT scan is the preferred modality in non-accidental brain trauma, MRI is done usually after 3–4 days to detect hypoxic brain injury by DWI (which is better than CT), thin subdural hematoma, or multiple petechial subcortical hemorrhages by adding gradient SWI sequences and also FLAIR. Sagittal T2W imaging of the cervical spine is also recommended to evaluate brainstem or cord injury which could be the result of violent shaking, in turn causing hypoxic injury.[48-50]
Children presenting with global developmental delay (GDD)/intellectual disability
Pediatric neurologist very often comes across children presenting with GDD. The first investigation offered is MRI. This is because MRI is found abnormal in almost 54.7% of cases with common structural abnormality seen in white matter, corpus callosum, and ventricles. In 39.6% of cases, MRI is helpful in clinching the right diagnosis either directly or indirectly which can be confirmed by other investigations. The protocols used are Axial DWI, TSE T1W, TSE T2W, FLAIR, PD, T2TIRM, coronal T1TIR, and T2 TSE and sagittal T1 TSE.[51] For initial screening, these protocols can be used but should be modified according to the findings. For example, if there is hydrocephalus or leukodystrophy, then appropriate additional sequences should be added. Thus, it is very important to look at the imaging findings during the scan to modify the protocols to avoid redo or repeat imaging.
Disease-specific recommended MRI protocols are summarized in [Table 1].
| Indications | MRI protocol |
|---|---|
| Myelination | SE T1W axial/sagittal Or 3D T1 MP-RAGE SE T2W axial/coronal Or dual-echo STIR axial/coronal FLAIR axial or 3D FLAIR |
| Routine brain | FLAIR axial or 3D FLAIR 3D T1 MP-RAGE or T1W SE axial/sagittal DWI axial SE/FSE T2W axial/coronal Or dual-echo STIR axial/coronal SWI axial Post-contrast 3D T1 MP-RAGE T1W axial with MT Or T1W axial/sagittal/coronal |
| Orbit (thin slice 3 mm) | Dual-echo STIR or T2W FS axial/coronal/sagittal obliques T1W FS Coronal/axial Post-contrast T1W FS axial/coronal |
| Pituitary (thin slice 3 mm) | Dual-echo STIR or T2W coronal/sagittal T1W sagittal/coronal Post-contrast T1W sagittal/coronal |
| Inner ear | Heavily T2W sequence like CISS 3D or FIESTA or T2 DRIVE |
| Tumor | Routine brain sequences with contrast FS SE T1W sagittal of whole spine MR spectroscopy Perfusion |
| Infection | Routine brain sequences with contrast Post-contrast FLAIR axial Post-contrast T1W with MT Heavily T2W sequence like CISS 3D MR spectroscopy MR perfusion |
| Hydrocephalus | Routine brain sequences 3D T2 SPACE Heavily T2W sequences like CISS 3D CSF flow study |
| Metabolic disease/leukodystrophy | Routine brain sequences T2 sagittal spine MR spectroscopy |
| Epilepsy | 3D FLAIR or FLAIR axial/coronal 3D T1W MP-RAGE or T1 sagittal/axial/ coronal 3D T2W SPACE or T2W/STIR/IR T1W axial/coronal DWI axial SWI axial |
| Demyelination | Routine brain sequences Orbital sequences FLAIR sagittal T2W sagittal spine Post-contrast axial 3D FLAIR or FLAIR axial/sagittal, T1W axial with MT, and T1W FS sagittal spine and coronal for orbits |
| Non-traumatic brain hemorrhage | Routine brain sequences MR angiogram MR venogram SWI axial |
| Stroke | Routine brain sequences MR angiogram of neck and brain Dual-echo STIR and FS T1W axial of neck |
| Non-accidental head trauma | Routine brain sequences with SWI T2 sagittal cervical spine |
| Global developmental delay | Axial FSE T1W, T2W, PD FLAIR DWI IR T2W Sagittal FSE T1W Coronal IR T1W, T2W FSE, FLAIR Or 3D MPRAGE/3D FLAIR Additional sequences depending upon the initial findings |
FS: Fat saturated, MT: Magnetization transfer, IR: Inversion recovery, SWI: Susceptibility weighted imaging, STIR: Short tau inversion recovery, SE: Spin echo, FSE: Fast spin echo, MP-RAGE: Magnetization prepared rapid gradient echo, CISS: Constructive interference in steady-state, PD: Proton density
CONCLUSION
Routine conventional imaging is sufficient to arrive at the diagnosis most times; however, a few advanced imaging techniques particularly functional MRI and spectroscopy add value. Physicians should be aware of these MRI sequences and should specifically include them in the request form, depending on their clinical diagnosis. This will save time and the need for repeat studies in children.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Comparison of neonatal MRI examinations with and without an MR-compatible incubator: Advantages in examination feasibility and clinical decision-making. Eur J Paediatr Neurol. 2010;14:410-7.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a nurse-led sedation service for paediatric magnetic resonance imaging. Lancet. 1999;56:388-91.
- [CrossRef] [Google Scholar]
- The management of infants and children for painless imaging. Clin Radiol. 2005;60:731-41.
- [CrossRef] [PubMed] [Google Scholar]
- Propofol-based sedation regimen for infants and children undergoing ambulatory magnetic resonance imaging. Br J Anaesth. 2008;101:239-43.
- [CrossRef] [PubMed] [Google Scholar]
- Anaesthesia or sedation for MRI in children. Curr Opin Anaesthesiol. 2010;23:513-7.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of dexmedetomidine with propofol for magnetic resonance imaging sleep studies in children. Anesth Analg. 2009;109:745-53.
- [CrossRef] [PubMed] [Google Scholar]
- Improved pediatric MR imaging with compressed sensing. Radiology. 2010;256:607-16.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in pediatric body MRI. Pediatr Radiol. 2011;41:549-54.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging protocols for paediatric neuroradiology. Pediatr Radiol. 2007;37:789-97.
- [CrossRef] [PubMed] [Google Scholar]
- Contrast-enhanced magnetic resonance imaging in pediatric patients: Review and recommendations for current practice. Magn Reson Insights. 2013;6:95-111.
- [CrossRef] [PubMed] [Google Scholar]
- Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol. 2008;43:817-28.
- [CrossRef] [PubMed] [Google Scholar]
- European Medicines Agency Makes Recommendations to Minimise risk of Nephrogenic Systemic Fibrosis with Gadolinium-Containing Contrast Agents, EMEA Press Office 2013. Available from: http://www.ema.europa.eu/docs/en_gb/document_library/press_release/2009/11/wc500015569.pdf [Last accessed on 2013 Jul 25]
- [Google Scholar]
- Nephrogenic systemic fibrosis and gadolinium-based contrast media: Updated ESUR contrast medium safety committee guidelines. Eur Radiol. 2013;23:307-18.
- [CrossRef] [PubMed] [Google Scholar]
- FDA Drug Safety Communication: New Warnings for Using Gadolinium-Based Contrast Agents in Patients with Kidney Dysfunction. 2013. Washington, DC: U.S. Department of Health and Human Services; Available from: http://www.fda.gov/drugs/drugsafety/ucm223966 [Last accessed on 2013 Jul 25]
- [Google Scholar]
- Safety and efficacy of gadobutrol-enhanced MRI in patients aged under 2 years-a single-center, observational study. Magn Reson Insights. 2013;6:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol. 2000;21:1099-109.
- [Google Scholar]
- Pediatric Neuroimaging (4th ed). Philadelphia, PA: Lippincott Williams & Wilkins; 2005. Ch. 1
- [Google Scholar]
- Normal myelination of the pediatric brain imaged with fluid-attenuated inversion-recovery (FLAIR) MR imaging. Am J Neuroradiol. 1999;20:1406-11.
- [Google Scholar]
- Changes in brain water during the first year of life. Radiology. 2002;222:405-9.
- [CrossRef] [PubMed] [Google Scholar]
- A modified inversion recovery sequence for routine high-contrast brain imaging (abstract) In: Abstract book of the Society of Magnetic Resonance Eighth Annual Meeting and Exhibition. Vol 2. New York: Wiley; 1989. p. :722.
- [Google Scholar]
- A protocol for imaging paediatric brain tumours. United Kingdom Children's cancer study group and societe francaise d'oncologie pediatrique panelists. Clin Radiol. 1999;54:558-62.
- [CrossRef] [Google Scholar]
- Conventional and advanced MRI features of pediatric intracranial tumors: Supratentorial tumors. AJR Am J Roentgenol. 2013;200:W483-503.
- [CrossRef] [PubMed] [Google Scholar]
- Surveillance neuroimaging in childhood intracranial ependymoma: How effective, how often, and for how long? J Neurosurg. 2001;94:27-32.
- [CrossRef] [PubMed] [Google Scholar]
- Surveillance neuroimaging of intracranial medulloblastoma in children: How effective, how often, and for how long? J Neurosurg. 2003;99:280-6.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of contrast-enhanced fluid-attenuated inversion recovery MR imaging of intracranial metastases. AJNR Am J Neuroradiol. 2004;25:761-5.
- [Google Scholar]
- Utility of contrast-enhanced fluid-attenuated inversion recovery in magnetic resonance imaging of intracranial lesions. West Afr J Radiol. 2018;25:34-8.
- [CrossRef] [Google Scholar]
- Different MRI sequences and spectrum of findings in pediatric brain infections in North Indian population. Int J Contemp Med Surg Radiol. 2019;4:D124-7.
- [CrossRef] [Google Scholar]
- Role of MRI in evaluation of ring enhancing lesions of brain in correlation with MR spectroscopy. Int J Contemp Med Surg Radiol. 2018;3:C33-7.
- [CrossRef] [Google Scholar]
- Perfusion-weighted imaging in differentiating ring-enhancing lesions in brain. J NTR Univ Health Sci. 2019;8:162-9.
- [CrossRef] [Google Scholar]
- Verification of brain ring enhancing lesions by advanced MR techniques. Alex J Med. 2018;54:167-71.
- [CrossRef] [Google Scholar]
- Role of 3D SPACE sequence and susceptibility weighted imaging in the evaluation of hydrocephalus and treatment-oriented refined classification of hydrocephalus. Indian J Radiol Imaging. 2018;28:385-94.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebrospinal fluid flow imaging by using phase-contrast MR technique. Br J Radiol. 2011;84:758-65.
- [CrossRef] [PubMed] [Google Scholar]
- Proton MR spectroscopy in leukodystrophies. Egypt J Radiol Nucl Med. 2015;46:1091-7.
- [CrossRef] [Google Scholar]
- Paediatric acquired demyelinating syndromes: Incidence, clinical and magnetic resonance imaging features. Mult Scler. 2013;19:76-86.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative magnetic resonance characterization of mesial temporal sclerosis in childhood. Neurology. 2001;56:1659-65.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia. 2009;50:2147-53.
- [CrossRef] [PubMed] [Google Scholar]
- 3T MRI Leads to Better Diagnosis for Focal Epilepsy, Study Suggests; Science Daily Spring City, Pennsylvania: American Roentgen Ray Society; 2008.
- [Google Scholar]
- MR imaging of epilepsy: Strategies for successful interpretation. Neuroimaging Clin N Am. 2004;14:349-72.
- [CrossRef] [PubMed] [Google Scholar]
- Is there a role for magnetic resonance imaging in the evaluation of non-traumatic intraparenchymal haemorrhage in children? Pediatr Radiol. 2006;36:940-6.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid brain MRI protocols reduce head computerized tomography use in the pediatric emergency department. BMC Pediatr. 2020;20:14.
- [CrossRef] [PubMed] [Google Scholar]
- One-minute ultrafast brain MRI with full basic sequences: Can it be a promising way forward for pediatric neuroimaging? AJR Am J Roentgenol. 2020;215:198-205.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging of the brain in pediatric patients: Diagnostic value of HASTE sequences. AJR Am J Roentgenol. 2002;179:509-14.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167-73.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior circulation stroke in childhood: Risk factors and recurrence. Neurology. 2002;59:1552-6.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid sequence MRI protocol in the evaluation of pediatric brain attacks. Pediatr Neurol. 2020;107:77-83.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroimaging for non-accidental head injury in childhood: A proposed protocol. Clin Radiol. 2003;58:44-53.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropathology of inflicted head injury in children. I. Patterns of brain damage. Brain. 2001;124:1290-8.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain. 2001;124:1299-306.
- [CrossRef] [PubMed] [Google Scholar]
- MRI evaluation of global developmental delay: A retrospective study. Dubai Med J. 2020;3:1-4.
- [CrossRef] [Google Scholar]






