Translate this page into:
New-onset discoid lupus erythematosus after Oxford-AstraZeneca adenoviral (Covishield™) vaccination in a paediatric patient
*Corresponding author: Sharang Gupta, Department of Dermatology, Government Medical College, Patiala, Punjab, India. drsharanggupta97@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Malhan S, Gupta S, Chopra D, Chopra P. New-onset discoid lupus erythematosus after Oxford-AstraZeneca adenoviral (Covishield™) vaccination in a paediatric patient. Karnataka Paediatr J. 2025;40:32-5. doi: 10.25259/KPJ_39_2024
Abstract
Oxford Astra-Zeneca adenoviral vaccine (Covishield™) is a viral vector vaccine that uses an attenuated, non-replicating strain of Chimpanzee adenovirus as a vector to carry the spike glycoprotein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into human cells. Although highly effective against SARS-CoV-2 infection coronavirus disease 19 (COVID-19), COVID-19 vaccines have been implicated in triggering and exacerbating various autoimmune diseases due to their role in inciting antigen-specific immune responses. There have been various reports of the development or exacerbation of dermatomyositis and systemic lupus erythematosus (LE) following COVID-19 vaccine. Here, we present the rare case of the development of discoid LE (DLE) in a 14-year-old female patient 3 weeks following the administration of the first dose of Covishield™ (ChAdOx1 nCoV-19 vaccine). The patient also had positive anti-nuclear antibody titres. The temporal relationship of her symptoms after vaccine administration could suggest a possible association between the vaccine and the development of DLE, as this is the time interval for antibody formation post-vaccine.
Keywords
Discoid lupus erythematosus
Paediatric discoid lupus erythematosus
Covishield™
Vaccine-induced lupus
INTRODUCTION
The term lupus erythematosus (LE) refers to a broad range of clinical conditions that are related to the development of an autoimmune response, primarily directed against the molecular components of nucleosomes and ribonucleoproteins. The cutaneous manifestations of LE can be roughly divided among lesions that exhibit the distinctive histologic alterations associated with LE, such as interface dermatitis (LE-specific skin disease), and those that do not (LE non-specific skin disease).[1] Acute cutaneous LE, subacute cutaneous LE, and chronic cutaneous LE (CCLE) are the three main subtypes of LE-specific skin diseases. Discoid LE (DLE) is a subtype of CCLE that includes classic DLE, hypertrophic DLE, and mucosal DLE, among its many morphological variants.
According to the literature, systemic lupus erythematosus (SLE) patients are more likely to experience adverse outcomes with severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) infection coronavirus disease 19 (COVID-19), and immunisation is recommended for SLE patients, particularly those receiving strong immunosuppressive medications.[2,3] However, given their role in eliciting antigen-specific immune responses and the fact that multiple cases of SLE have been documented following SARS-CoV2-vaccination,[2,4-8] vaccines have been considered a potential trigger for the development of SLE. Various cases of SLE exacerbations following SARS-CoV2 vaccination have been reported, including the relapse of class V (membranous) lupus nephritis.[8-10] However, we could not identify any reports describing the development of DLE after SARS-CoV2-vaccination. We report the case of a 14-year-old female who developed clinical symptoms of DLE 3 weeks after administration of the first dose of the recombinant Oxford-AstraZeneca adenoviral vaccine (Covishield™).
CASE REPORT
A 14-year-old Indian female without any history of autoimmune disorders presented to the dermatology outpatient department of a tertiary care hospital with well-defined, scaly and erythematous to violaceous plaques on the dorsal as well as palmar aspect of bilateral hands for 1.5 months. The lesions showed characteristic surrounding hyperpigmentation [Figure 1]. This was followed by the appearance of hyperpigmented plaques on the concha of bilateral ears [Figure 2]. The patient had received the first dose of Covishield™ (ChAdOx1 nCoV-19 vaccine) 3 weeks before the onset of lesions. There was no history of preceding trauma before the onset of lesions. The rest of the cutaneous examination was grossly unremarkable. There was no prior history of joint pains, malar rash or photosensitivity. No significant family history of autoimmune illnesses was noted. A dermoscopy of the lesion on the right hand revealed cicatricial milky red areas, structureless white patches and a peripheral pigmented network [Figure 3]. Disease activity was assessed using the cutaneous LE disease area and severity index (CLASI) score. The CLASI-A (activity score) was 6, indicating a mild disease activity at presentation.
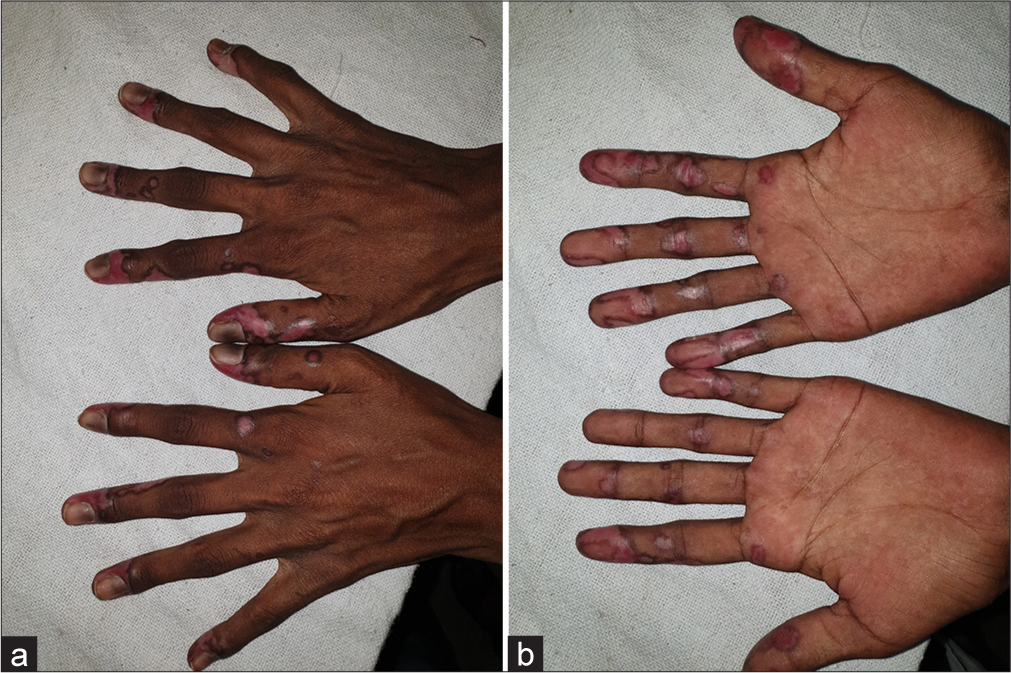
- (a) Well-defined, erythematous to violaceous scaly plaques with surrounding hyperpigmentation on the dorsal and (b) ventral surface of bilateral hands.
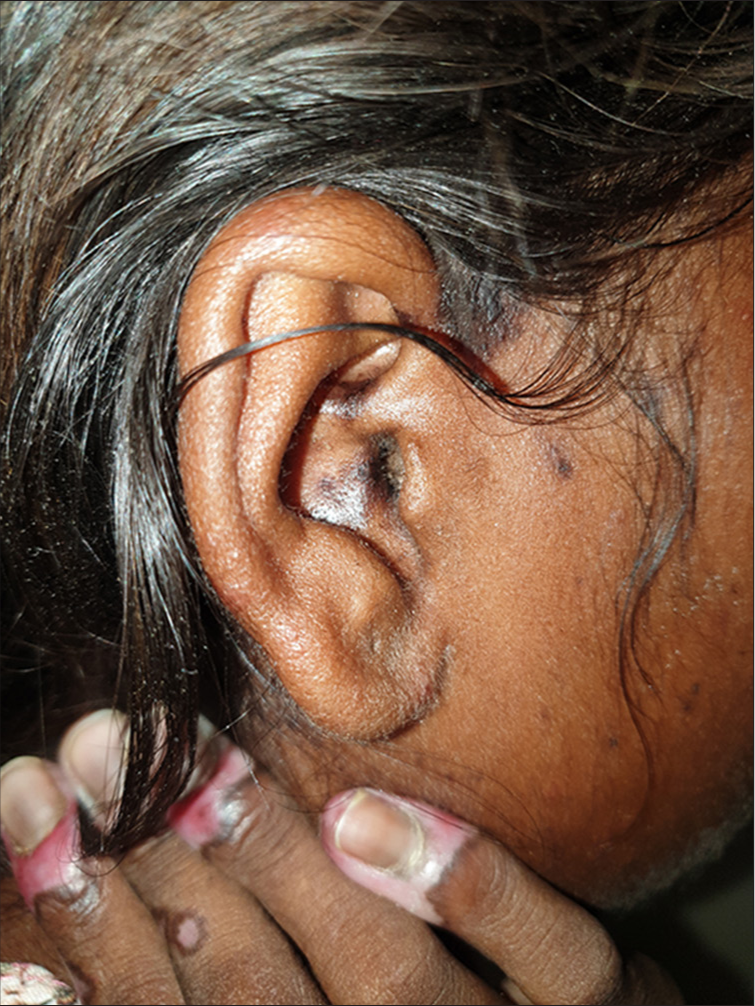
- Hyperpigmented plaque with follicular plugging involving the concha of the ear.
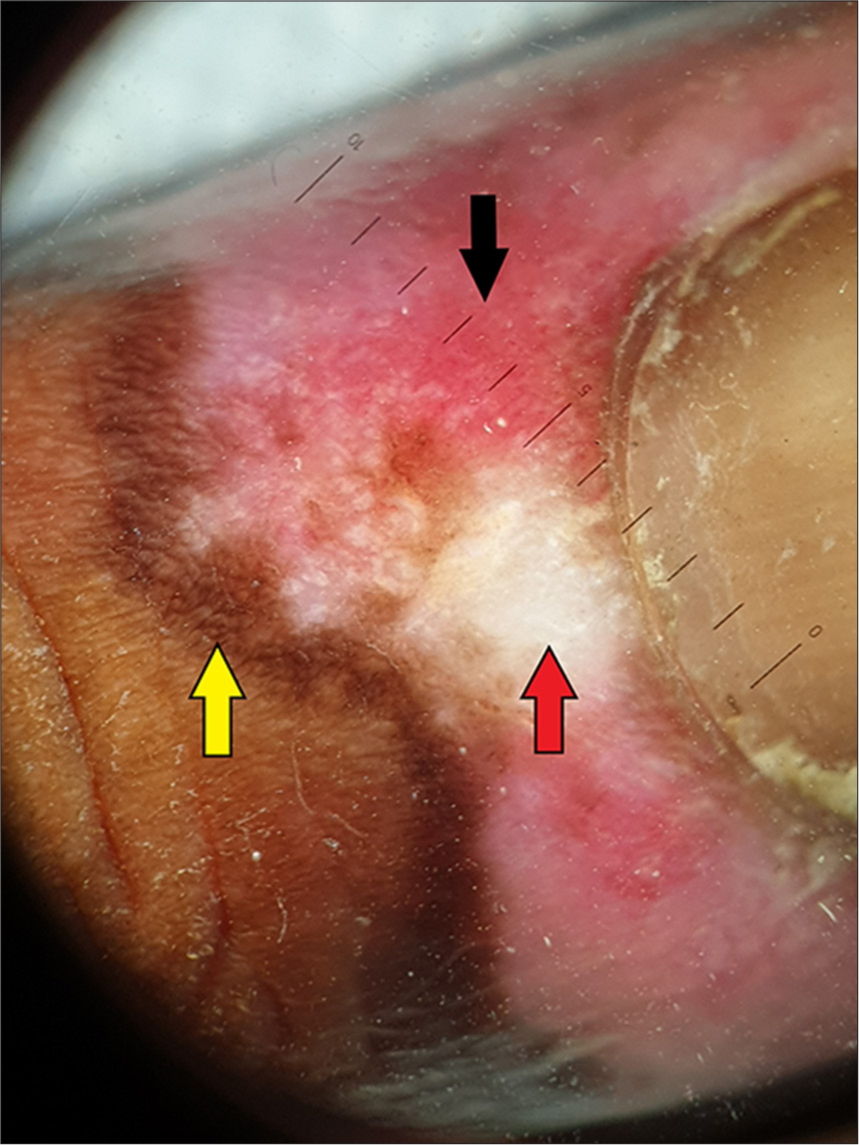
- Dermoscopy of the lesion on the right hand showing cicatricial milky red areas (black arrows), structureless white patches (red arrow) and a peripheral pigmented network (yellow arrow) (Heine Delta 20T dermatoscope, non-polarised contact mode, ×10).
Laboratory investigations revealed an elevated anti-nuclear antibody titre (1:160). Based on the above findings, a formal diagnosis of DLE was made. Other serological investigations such as anti-Sjögren’s-syndrome-related antigen A (anti-SSA), anti-Sjögren’s-syndrome-related antigen B(anti-SSB), Anti-double-stranded deoxyribonucleic acid (anti-dsDNA), anti-Smith (anti-Sm), and anti-ribonucleoprotein (anti-RNP) could not be done due to the financial constraints of the patient. The patient was counselled to undergo a skin biopsy, but did not consent. The patient was lost to follow-up after the initial investigations.
DISCUSSION
DLE is a subtype of CCLE that is frequently characterised by lesions on the photo-exposed parts of the body. It is more common in the second to fourth decade of life, and the female-to-male ratio ranges between 3:2 and 3:1, which is considerably lower than that of SLE.[11] Pathogenesis of this disease is multi factorial and involves an interplay of various host and environmental factors. In genetically predisposed individuals, environmental factors such as ultraviolet radiation, certain drugs, and infections can cause cell damage, thereby activating the immune response. Autoantibodies and immune complexes are formed and deposited as a result of an inflammatory cascade of cytokine activation, specifically the type I and type II interferons.[12] Comprehensive research has been done on the role of viral infections, such as cytomegalovirus, hepatitis A, and coronaviruses, in initiating or aggravating autoimmune disorders. Molecular mimicry, bystander inflammation, epitope spreading, and the exposure of cryptic antigens are some of the potential mechanisms at play.[13]
It is a well-known fact that the COVID-19 virus can trigger or exacerbate autoimmune disorders; however, it is noteworthy that in certain cases, these disorders are triggered or exacerbated by the vaccine itself. Covishield ™ is a viral vector vaccine that uses an attenuated, non-replicating strain of chimpanzee adenovirus as a vector to carry the spike glycoprotein of SARS-CoV-2 into human cells.[14] On the administration of the Covishield™ vaccine, T-cells are activated, which initiates an immune response. A rise in CD8 T-cell count triggers the activation of B-cells, which synthesize virus-neutralising antibodies. It takes around 2–3 weeks for antibodies to develop, and these are of immunoglobulin G type, which stays for an extended period.
Sprow et al. reported the exacerbation of symptoms in preexisting cases of dermatomyositis and SLE following the COVID-19 vaccine, although these exacerbations were significantly more common in dermatomyositis than in SLE.[15] A case series published by Garg et al. in 2022 reported six cases of new-onset cutaneous autoimmune disorders and four cases of aggravation of pre-existing disorders following Covishield™ vaccine. According to this case series, Covishield™ has also been reported to trigger alopecia areata, lichen planus, vitiligo with leukotrichia and exacerbate previously diagnosed cases of vitiligo and lichen planus.[13]
Autoimmunity induced by inactivated vector vaccines is currently being rigorously studied. Other vaccines with inactivated viral vector delivery, such as influenza vaccine, have also been linked to the flaring of autoimmune diseases in recipients,[16] thereby implicating a role of viral vector in eliciting host immune response. However, in a study done by Kanduc et al., significant peptide sharing was seen between SARS-CoV-2 spike protein and human proteins at the heptapeptide level, suggesting that antibodies produced in response to the spike protein may cross-react with the host and induce autoimmune responses.[16]
In patients with cutaneous LE, increased levels of Th1 cells and Th 1- Th1-associated cytokines have been reported in lesional skin following SARS-CoV-2 vaccination.
Although exacerbation of pre-existing cutaneous autoimmune disorders has been reported following the COVID-19 vaccine, reports of the development of cutaneous autoimmune disorders following the COVID-19 vaccine in previously healthy patients are rare. We report herein a case of a 14-year-old female who developed DLE-like lesions on bilateral hands and concha of bilateral ears 3 weeks following the administration of the first dose of the Covishield™ vaccine.
The period between vaccine administration and the development of lesions is consistent with the time interval for antibody formation to occur following vaccination, thereby indicating the possible role of vaccine-triggered autoimmunity. The CLASI, a tool designed to score the disease activity (the presence of erythema, scale and hypertrophy) and damage (dyspigmentation, scarring and atrophy), can be used by healthcare providers to assess the skin lesions and a thorough systemic examination should also be done to look for signs and symptoms of systemic LE.
However, the possibility that the development of these lesions was merely a coincidence and not associated with vaccine administration cannot be completely ruled out. In a study done by Mok et al. to determine the hesitancy for SARSCoV-2 vaccines and post-vaccination flares in patients with SLE, it was observed that 8.2% of the vaccinated patients with SLE experienced flares, which was not statistically significant compared with the 6.2% flare rate in unvaccinated SLE controls. Identification of flare associated risk factors such as positive lupus serology and a history of arthritis or discoid lesions before vaccination can help us in predicting post-vaccination flare risk and in selecting the right candidates for vaccination.[17]
It is also possible that the patient had a positive lupus serology (anti-nuclear antibodies [ANA] titres) before receiving the vaccine and therefore was already at risk of developing DLE, which was triggered upon vaccine administration. Since the pre-vaccination serology of the patient is not known, this possibility cannot be completely ruled out.
The existing literature suggests that the severity of signs and symptoms of autoimmune conditions is further exacerbated after receiving the second dose of the COVID-19 vaccination or with prior COVID-19 infection.[12] This suggests that the enhanced immune response brought on by repeated exposure to either the vaccine or the virus itself corresponds to an enhanced autoimmune response and raises the prospect that booster doses may also increase the risk of autoimmune exacerbations. However, disease activity following subsequent doses of the vaccine could not be evaluated in this patient due to loss of follow-up.
However, vaccine-induced autoimmune illnesses are extremely rare and should not overshadow the advantages of immunisation, especially in those who are vulnerable.
CONCLUSION
COVID-19 vaccines can trigger new-onset autoimmune diseases and exacerbate pre-existing autoimmune diseases in recipients. This case report defines the rare case of new-onset DLE in a paediatric patient following Covishield™ (ChAdOx1 nCoV-19 vaccine). However, these side effects are rare and should not outweigh the benefits of these vaccines, especially in vulnerable individuals.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol. 1981;4:471-5.
- [CrossRef] [PubMed] [Google Scholar]
- Lupus, vaccinations and COVID-19: What we know now. Lupus. 2021;30:1541-52.
- [CrossRef] [PubMed] [Google Scholar]
- Influenza virus vaccination of patients with SLE: Effects on generation of autoantibodies. Clin Rheumatol. 2002;21:369-72.
- [CrossRef] [PubMed] [Google Scholar]
- Local and systemic reactogenicity of COVID-19 vaccine BNT162b2 in patients with systemic lupus erythematosus and rheumatoid arthritis. Rheumatol Int. 2021;41:1925-31.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic lupus erythematosus after COVID-19 vaccination: A case report. J Cosmet Dermatol. 2021;20:3103-4.
- [CrossRef] [PubMed] [Google Scholar]
- First presentation of systemic lupus erythematosus in a 24-year-old male following mRNA COVID-19 vaccine. Case Rep Rheumatol. 2022;2022:9698138.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic lupus erythematous after Pfizer COVID-19 vaccine: A case report. Clin Rheumatol. 2022;41:1597-601.
- [CrossRef] [PubMed] [Google Scholar]
- Relapse of class V lupus nephritis after vaccination with COVID-19 mRNA vaccine. Kidney Int. 2021;100:941-4.
- [CrossRef] [PubMed] [Google Scholar]
- Vaccinations and risk of systemic lupus erythematosus and rheumatoid arthritis: A systematic review and meta-analysis. Autoimmun Rev. 2017;16:756-65.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of immune response and disease status in systemic lupus erythematosus patients following SARS-CoV-2 vaccination. Arthritis Rheumatol. 2022;74:284-94.
- [CrossRef] [PubMed] [Google Scholar]
- Population-based prevalence and incidence estimates of primary discoid lupus erythematosus from the Manhattan Lupus Surveillance Program. Lupus Sci Med. 2019;6:e000344.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology of cutaneous lupus erythematosus. Arthritis Res Ther. 2015;17:182.
- [CrossRef] [PubMed] [Google Scholar]
- Is covid vaccine (covishield) giving rise to de novo cutaneous autoimmune disorders and aggravating the existing ones: A case series of 10 patients with review of literature. Med Res Arch. 2022;10:10.
- [CrossRef] [Google Scholar]
- COVID-19 vaccine: A comprehensive status report. Virus Res. 2020;288:198114.
- [CrossRef] [PubMed] [Google Scholar]
- Autoimmune skin disease exacerbations following Covid-19 vaccination. Front Immunol. 2022;13:899526.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular mimicry between SARSCoV-2 spike glycoprotein and mammalian proteomes: Implications for the vaccine. Immunol Res. 2020;68:310-3.
- [CrossRef] [PubMed] [Google Scholar]
- Hesitancy for SARS-CoV-2 vaccines and post-vaccination flares in patients with systemic lupus erythematosus. Vaccine. 2022;40:5959-64.
- [CrossRef] [PubMed] [Google Scholar]







