Translate this page into:
An observational study of salivary C-reactive protein as a potential novel non-invasive biomarker of neonatal sepsis
*Corresponding author: Sanjeev B. Rai, MD, Department of Paediatrics, Father Muller Medical College, Mangalore, Karnataka, India. raibs11@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Murugesan I, Rai SB. An observational study of salivary C-reactive protein as a potential novel non-invasive biomarker of neonatal sepsis. Karnataka Paediatr J 2021;36:123-8.
Abstract
Objectives:
Serial C-reactive protein (CRP) monitoring helps to rule out and prognosticate sepsis. Small blood volumes in neonates make it difficult for repeated blood draws for serial CRP monitoring. Hence, the need of the hour is a non-invasive method such as CRP estimation in saliva. This study aims to correlate salivary CRP with serum CRP levels and establish the potential clinical utility of salivary CRP in diagnosing neonatal sepsis.
Materials and Methods:
Twenty-three consecutive neonates diagnosed with clinically suspected sepsis and admitted to the NICU were the study subjects. Demographics such as gestational age and weight at birth, sex and detailed clinical features, and comorbidities were noted. Blood samples for CRP estimation and blood culture were collected as soon as clinical suspicion of sepsis arose. Saliva samples were collected for CRP estimation within 1 h of blood sample collection. The saliva was collected in a 2 mL syringe using low suction. Salivary and serum CRP were estimated by the particle enhanced immunoturbidimetric assay.
Results:
In our study, the CRP levels in saliva correlated moderately well with CRP levels in serum (Spearman correlation coefficient r = 0.582, P = 0.004). The sensitivity and specificity of salivary CRP to predict a serum level of ≥10 mg/L were observed to be 0.75 and 0.93, respectively.
Conclusion:
Our study shows the promise of salivary CRP as a potential clinically meaningful biomarker of neonatal sepsis and warrants the need for larger studies to validate the utility of salivary CRP to serially monitor neonatal sepsis.
Keywords
Neonatal sepsis
Non-invasive tool
Salivary C-reactive protein
Serum C-reactive protein
Biomarker
INTRODUCTION
Since sepsis is the number one cause of neonatal mortality in India, effective diagnostic tools are of paramount importance.[1] Neonatal sepsis is often diagnosed by clinical signs and symptoms that are non-specific and incapable of recognizing early-onset sepsis.[1-3] Blood culture is the current gold standard; however, results of microbial growth can take up to 2 days to be detected and are positive only in 25–40% of cases.[4] Thus, a culture-independent tool for sepsis diagnosis is the need of the hour. Previously, blood-derived serial C-reactive protein (CRP) measurements have been demonstrated to help rule out and prognosticate neonatal sepsis.[5] Nevertheless, due to limited blood volumes in neonates, repeated blood draws for serial CRP monitoring are not ideal. Studies have shown that CRP can also be estimated from the saliva.[6,7]
If so, non-invasive monitoring using saliva based CRP may be a feasible choice for the investigation of neonatal sepsis. Conclusive studies on the clinical utility of salivary CRP in identifying neonatal sepsis are lacking. Difficulty in collecting neonatal saliva samples, lack of studies about salivary CRP cutoff, comparison with blood culture, neonatal salivary flow dynamics, salivary proteins and confounding factors, comparison of different methods such as enzyme-linked immunosorbent assay (ELISA) and turbidimetry, and paucity of Indian studies are the reasons why salivary CRP has not yet been translated into clinical practice.
Iyengar et al. discovered a fair correlation between CRP in serum and saliva (r = 0.62, P < 0.001) and found that salivary CRP predicted serum CRP levels of ≥ 10 (AUC = 0.81) with a sensitivity and specificity 0.64 and 0.94, respectively. Around 50% of their study subjects were hospitalized for post-operative monitoring.[6] Omran et al. also found a fair correlation between salivary and serum CRP (0.66, P < 0.001). At a cutoff point of 3.48 ng/L, salivary CRP showed a sensitivity of 94% and specificity of 80%, but, they have restricted their study subjects to full-term neonates.[7] Our aim is to estimate the salivary CRP and serum CRP levels in clinically suspected early-onset neonatal sepsis and to study its utility in neonatal sepsis diagnosis. Unlike the previous studies, there is no exclusion based on gestational age and clinical features. ELISA kits were used in other studies, while in our study, immunoturbidimetry was used to determine salivary CRP, which is cost efficient and does not require additional apparatus or training.
MATERIALS AND METHODS
This study was conducted at a tertiary level medical college hospital after institutional ethical approval (IEC/ CCM/221/2019 Protocol No. 177/19 dated 19/08/2019). Written informed consent was obtained from the parents of the newborns. Neonates (age less than 28 days) hospitalized with clinical suspicion of sepsis were the study subjects. The inclusion and exclusion criteria for this study are elucidated below.
Inclusion criteria
Babies clinically suspected of neonatal sepsis
Babies whose CRP level was estimated.
Exclusion criteria
Babies suspected of sepsis but CRP levels were not estimated
Babies already on antibiotics before admission
Neonates with major congenital abnormalities, oral infections.
Sample size estimation
α (two tailed) = 0.05; β = 0.10; r = 0.62 (r = anticipated correlation coefficient.[8] Iyengar et al. obtained a coefficient of 0.62. Datla et al. obtained a similar coefficient of 0.63[6,9]).
Twenty-three random neonates that satisfied the inclusion-exclusion criteria were subsequently taken up for the study by purposive sampling technique. Demographics such as gestational age and weight at birth, sex and detailed clinical features, intrapartum events, treatment history, and day of presentation of symptoms were noted. When clinical suspicion of sepsis arose, complete sepsis screen was done. Blood samples for complete blood count, serum CRP, and blood culture were collected. Salivary samples were collected within 1 h of clinically indicated serum CRP measurement. For neonates receiving oral feeds, saliva was collected 1 h before a feed to avoid breast milk or formula contamination.
For saliva sample collection, a 2 mL syringe without plunger and end caps was placed under the tongue and attached to low suction to be sure that the saliva sample retains in syringe and does not go into the trap or tube of the suctioning apparatus.[6,10] Care was taken to keep suction pressure low to prevent mucosal bleed and contamination of saliva sample. The minimum volume of saliva required was 250 μL. The saliva sample was transferred to a vial and centrifuged at 3000 rpm for 15 min and CRP level was estimated immediately. Simultaneously, the blood sample was collected for CRP and blood culture; BD BACTEC automated blood culture system was used.
Principle of estimation of serum and salivary CRP
The particle enhanced immunoturbidimetric assay was used.[11] Human CRP can agglutinate monoclonal anti-CRP antibodies which are present on latex particles. Once the precipitate is obtained, the CRP level is estimated turbidimetrically on a Roche/Hitachi Cobas c system after careful calibration and quality control measures (CRPLX: ACN 019 – In vitro test for the quantitative determination of CRP; measuring range: 1.00–250 mg/L). The turbidimetric method was validated for use with human serum by the manufacturer. The same method was used for saliva without any methodological modifications. Quality control was employed using external and internal quality control programs of Bio-Rad, USA. The sample is mixed with an activation buffer and latex reagent (suspension of latex particles coated with anti-human CRP antibodies) and allowed to react. CRP in the sample reacts to form an insoluble complex, causing turbidity which is interpreted at 546 nm. More the turbidity, higher the concentration of CRP in the sample.[12] Automated analyser was used for obtaining the readings in case of both serum and saliva. The result is obtained in mg/L.
Data were analysed using IBM SPSS statistical package, version 21. Descriptive statistics were expressed as median ± interquartile range for numerical variables due to the skewed distribution of data and frequency and proportion for categorical variables. Spearman correlation coefficients with their 95% confidence intervals have been used to describe the correlation between CRP values in serum and saliva because the data points did not follow normality assumptions. The predictive power of salivary CRP to detect elevated serum levels of CRP (at two different serum level cutoffs of ≥5 mg/L and ≥10 mg/L) was studied by estimating the area under the receiver operator characteristic (ROC) curve and the salivary CRP value that maximized the Youden’s J statistic was chosen to report performance metrics such as sensitivity and specificity. P < 0.05 was considered statistically significant.
RESULTS
Out of 23 subjects, 16 were male and seven were female. About 17.4% (4/23 neonates) were culture-proven sepsis. The median gestational age and weight at birth were 38 weeks 5 days ± 2 weeks 1 day and 2920 ± 951 g, respectively. About 70% (16/23) of neonates presented with respiratory distress and 3 with jaundice. The remaining four babies presented with necrotizing enterocolitis (4.3%), intrauterine growth retardation (4.3%), features of meningitis (4.3%), and meconium aspiration (4.3%), respectively.
The median serum and salivary CRP values were 0.51 ± 12.51 mg/L and 9.30 ± 12.70 mg/L, respectively, as shown in [Table 1]. The coefficient of correlation between serum and salivary CRP was r = 0.582 with a significance of P = 0.004. This suggests a moderately good correlation, also supported by the graph shown in [Figure 1]. The neonates were binarily classified based on the serum CRP levels, CRP <5 mg/L as one group and CRP ≥5 mg/L as the other group. Since the data did not follow normality assumptions, Mann–Whitney U-test was done to establish that the difference in the average values of salivary CRP and was significant, as shown in [Table 2]. Sensitivity and specificity were determined for salivary CRP to differentiate a serum CRP level of ≥5 mg/L and an ROC curve was plotted [Figure 2], the AUC was estimated to be 0.754. A threshold of 14.8 mg/L that maximized the Youden’s J statistic was subsequently chosen as the salivary CRP cutoff [Figure 3]. At 14.8 mg/L, the sensitivity and specificity of salivary CRP to predict a serum CRP ≥5 mg/L were found to be 0.6 and 0.92, respectively [Table 3 and Figure 3].
| Serum CRP (mg/L) | |
|---|---|
| Mean | 15.8248 |
| Standard deviation | 8.78 |
| Median | 0.51 |
| Interquartile range | 12.51 |
| Range | 201.33 |
| Salivary CRP (mg/L) | |
| Mean | 15.4957 |
| Standard deviation | 3.79 |
| Median | 9.30 |
| Interquartile range | 12.70 |
| Range | 83.80 |
| Metric | No. of neonates in each group | Median salivary CRP (mg/L) with interquartile range | Significance (P-value) |
|---|---|---|---|
| Serum CRP<5 mg/L |
13 | 8.35±6.47 | 0.041 |
| Serum CRP≥5 mg/L |
10 | 16.5±20.74 | |
| Serum CRP<10 mg/L | 15 | 8.25±5.00 | 0.004 |
| Serum CRP≥10 mg/L | 8 | 19.35±27.71 |
| Metric | Sensitivity | Specificity | Area under ROC curve with (95% confidence intervals) | P-value |
|---|---|---|---|---|
| Differentiating a serum CRP level ≥5 mg/L at a salivary cutoff of 14.8 mg/L |
0.6 | 0.92 | 0.754 (0.543–0.965) | 0.041 |
| Differentiating a serum CRP level ≥10 mg/L at a salivary cutoff of 14.8mg/L |
0.75 | 0.93 | 0.875 (0.721–1.000) | 0.004 |
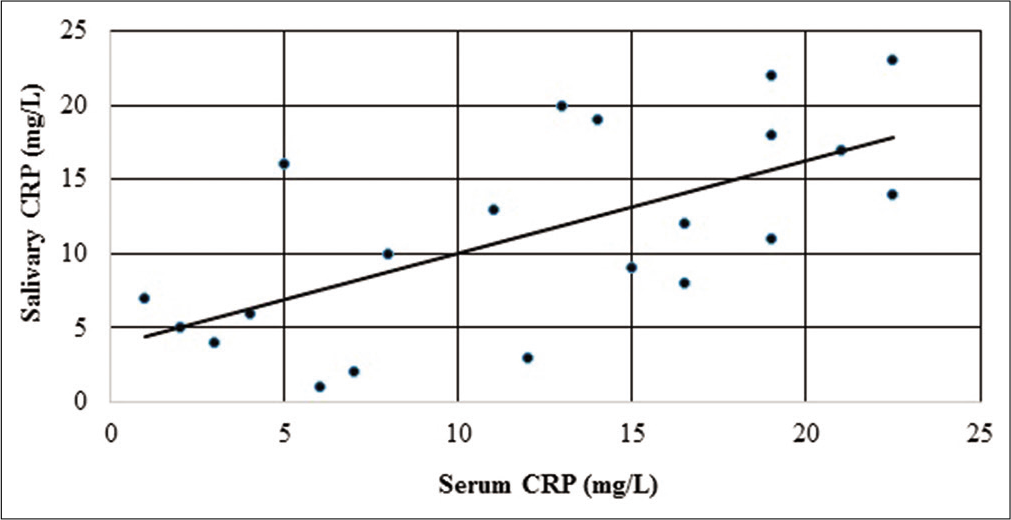
- Graph showing the correlation between serum CRP and salivary CRP levels after ranking (n = 23 neonates). Spearman’s rho was then calculated and was found to be rs = 0.582, P = 0.004.
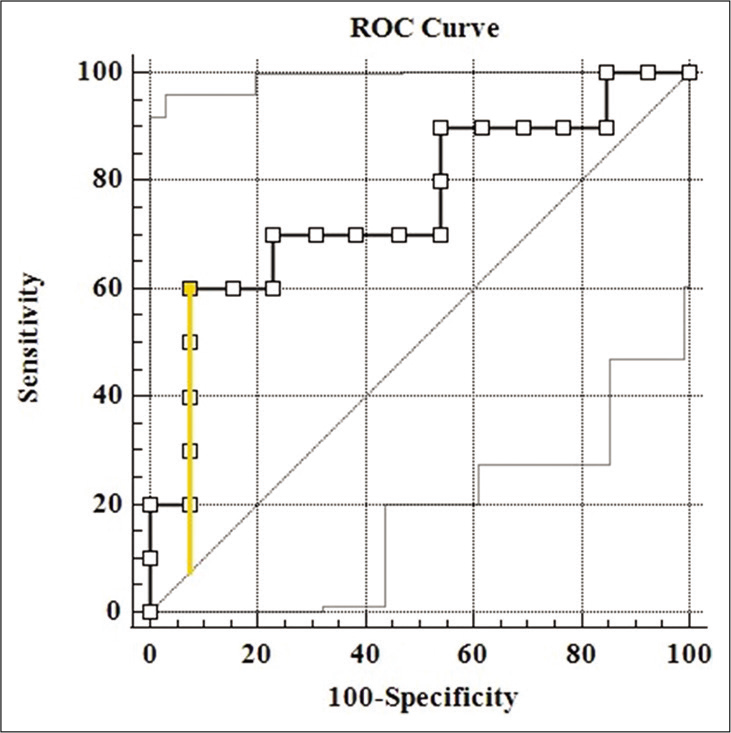
- ROC curve of salivary CRP to correctly tell a serum CRP level of 5 mg/L has been shown (AUC = 0.754, P = 0.041). The point corresponding to the Youden’s index has been marked and the intercept plotted.
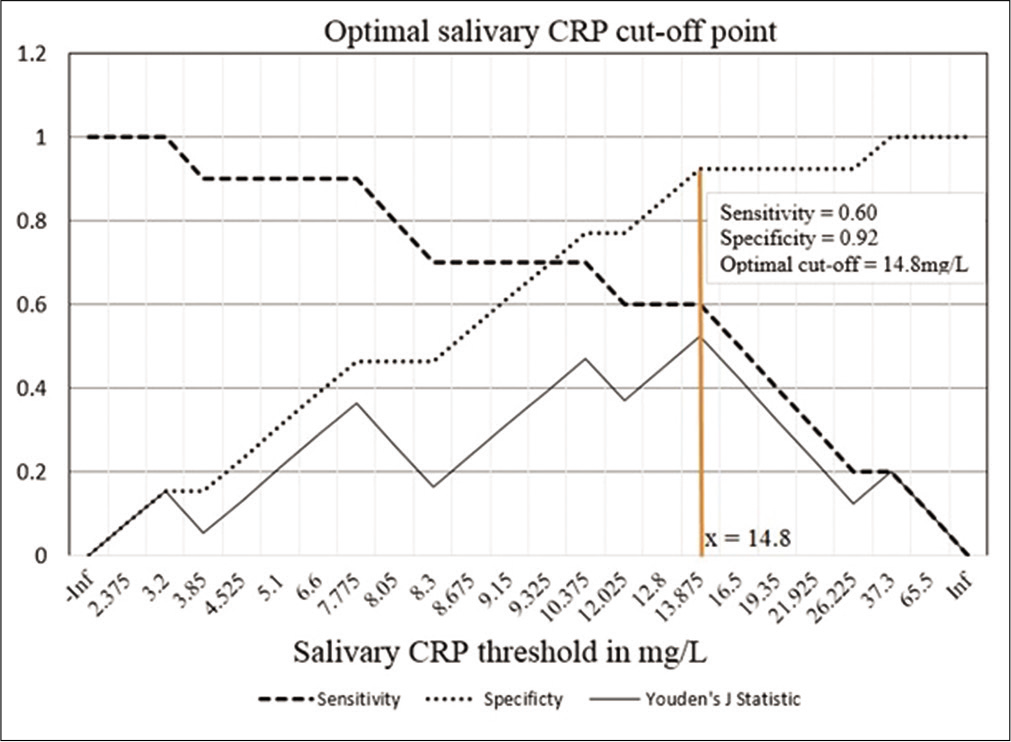
- Graph showing the sensitivity, specificity, and Youden’s J statistic over different salivary CRP thresholds. The salivary CRP cutoff that maximized the Youden’s J statistic has been shown. The corresponding sensitivity and specificity of salivary CRP to predict a serum CRP 5 mg/L were found to be 0.6 and 0.92, respectively
Next, the newborns were binarily classified based on the serum CRP levels of serum CRP <10 mg/L and serum CRP level ≥10 mg/L. Similarly, a Mann–Whitney U-test was done to identify a significant difference in the average salivary CRP values of both the groups (P = 0.004). Sensitivity and specificity were determined for salivary CRP to differentiate a serum CRP level of ≥10 mg/L and an ROC curve was plotted [Figure 4], the AUC was estimated to be 0.875. A threshold of 14.8 mg/L that maximized the Youden’s J statistic was subsequently chosen as the salivary CRP cutoff [Figure 5]. At 14.8 mg/L, the sensitivity and specificity of salivary CRP to predict a serum CRP ≥10 mg/L were found to be 0.75 and 0.93, respectively [Table 3 and Figure 5].
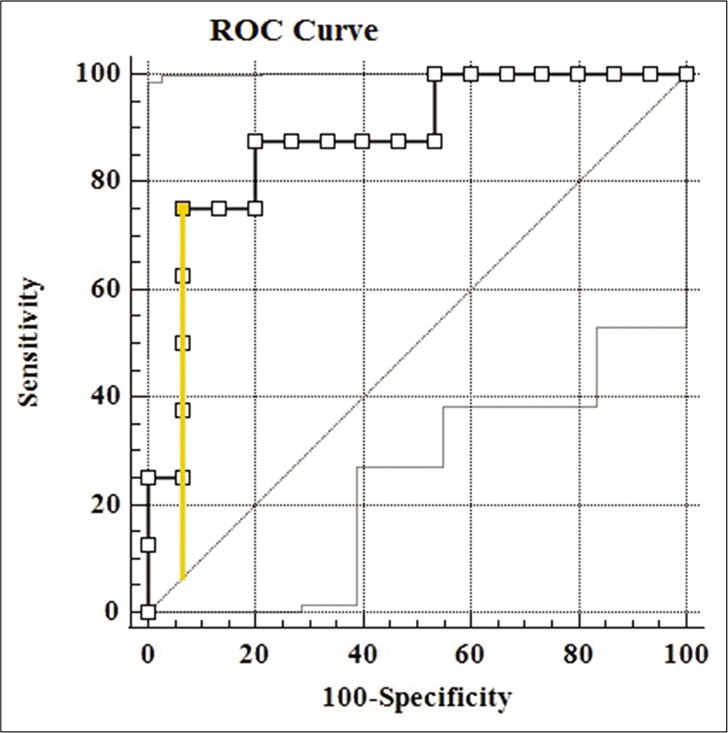
- ROC curve of salivary CRP to correctly tell a serum CRP level of 10 mg/L has been shown (AUC = 0.0.875, P = 0.004). The point corresponding to the Youden’s index has been marked and the intercept plotted.
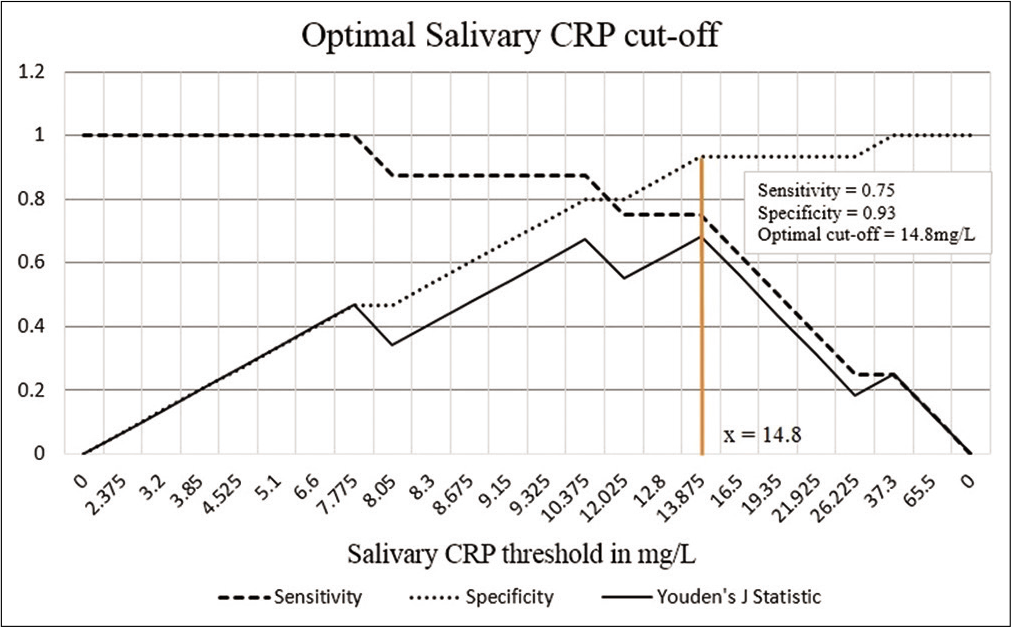
- Graph showing the sensitivity, specificity, and Youden’s J statistic over different salivary CRP thresholds. The salivary CRP cutoff that maximized the Youden’s J statistic has been shown. The corresponding sensitivity and specificity of salivary CRP to predict a serum CRP 10 mg/L were found to be 0.75 and 0.93, respectively.
DISCUSSION
About 30–50% of all neonatal deaths in developing countries are due to sepsis.[13] Early diagnosis and proper serial monitoring of disease progress is important in the clinical management of sepsis. At present, diagnostic guidelines for neonatal sepsis are based on constant clinical surveillance and serial serum CRP concentrations. Despite its non-specificity, serum CRP has been extensively used in neonatal sepsis due to its fast, cost effective, and simple determination.[7]
Our study had a good, positive relationship between serum and salivary CRP values and also establishes a salivary CRP cutoff point for diagnosing neonatal sepsis. Treatment was started immediately after sample collection and all the study subjects made a full recovery as of last follow-up.
The salivary CRP values and optimal cutoff obtained in our study were different from the previous studies. This can be explained by difference in the methodology and study subject characteristics. Studies on salivary CRP conducted in adults employing the turbidimetric method have expressed the results in mg/L. Corresponding studies in neonates are lacking.[12,14]
Unlike the previous studies, no additional external equipment was employed in the collection process, pre-processing, and final estimation of salivary CRP over serum CRP. To the best of the author’s knowledge, studies investigating particle enhanced immunoturbidimetry to estimate salivary CRP in neonatal sepsis could not be found on standard search. Serial salivary CRP monitoring, follow-up on recovery, and performance against blood culture were outside the scope of this study.
Realizing the importance of salivary CRP, rapid diagnostic tools are on the rise. Datla et al. employed a different salivary sample collection technique wherein a swab which when left in the mouth would soak up the required quantity of saliva for CRP estimation. This is less incidence of mucosal bleed with this method.[9] Novel techniques using a microchip assay system (sensitivity = 10 pg/mL), organic transistors, and smartphone-based immunoassays have been developed for rapid, accurate, and cost-effective estimation. Efforts are also being undertaken to convert ELISA and other photometric techniques to paper-based methods that can be interpreted using a smartphone.[15-17] The upcoming microfluidic immunosensor technology with higher sensitivity for CRP estimation seems to be a promising diagnostic tool.[18] Lin et al. demonstrated a correlation between serum and salivary CRP at different time points in neonatal sepsis and also established the mechanism of preferential movement of CRP across the blood saliva barrier using in vitro models of oral mucosa and salivary glands which strongly underlines the feasibility of salivary CRP as a non-invasive biomarker.[19]
CONCLUSION
Studies on the progression of salivary CRP levels with worsening illness and recovery, in babies with gastrointestinal intolerance, renal failure, and other comorbidities and studies on the timing and rate of change of salivary CRP with respect to serum CRP would be crucial.
A larger sample size prospective controlled study is further needed to determine the influence of various confounding factors on salivary secretion and sampling and importantly validate salivary CRP as potential routine standard of care diagnostic for neonatal sepsis.
Acknowledgments
We thank Dr. Shivashankara, Department of Biochemistry, Dr. Praveen B.K. and Dr. Saritha, Department of Paediatrics, and Mrs. Sucharitha Suresh, Department of Community Medicine, for their kind assistance. The authors are grateful to all the study participants, NICU staff, and Biochemistry Laboratory Staff for their cooperation.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
The study was supported by the Indian Academy of Paediatrics Research Award for medical undergraduates, awarded to Ms. Induparkavi Murugesan, undergraduate student.
Conflicts of interest
There are no conflicts of interest
References
- Risk factors of neonatal sepsis in India: A systematic review and meta-analysis. PLoS One. 2019;14:215683.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges in the diagnosis and management of neonatal sepsis. J Trop Pediatr. 2015;61:1-13.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of screening of neonatal sepsis. Int J Contem Pediatr. 2018;5:580-3.
- [CrossRef] [Google Scholar]
- Procalcitonin, C-reactive protein, and presepsin for the diagnosis of sepsis in adults and children. Cochrane Database Syst Rev. 2017;4:CD012627.
- [CrossRef] [Google Scholar]
- Detection and potential utility of C-reactive protein in saliva of neonates. Front Pediatr. 2014;2:131.
- [CrossRef] [PubMed] [Google Scholar]
- Salivary C-reactive protein, mean platelet volume and neutrophil lymphocyte ratio as diagnostic markers for neonatal sepsis. J Pediatr (Rio J). 2018;94:82-7.
- [CrossRef] [PubMed] [Google Scholar]
- Designing clinical research: An Epidemiologic Approach (4th ed). Philadelphia, PA: Lippincott Williams and Wilkins; 2013. p. :79.
- [Google Scholar]
- Diagnostic reliability of salivary C-reactive protein as an alternative noninvasive biomarker of neonatal sepsis. Indian Pediatr. 2021;28:S097475.
- [CrossRef] [PubMed] [Google Scholar]
- Optimal techniques for mRNA extraction from neonatal salivary supernatant. Neonatology. 2012;101:55-60.
- [CrossRef] [PubMed] [Google Scholar]
- Turbidimetric immunoassay of serum C-reactive protein. Clin Chem. 1982;28:2121-4.
- [CrossRef] [PubMed] [Google Scholar]
- Altered serum and salivary C-reactive protein levels in patients with oral premalignant lesions and oral squamous cell carcinoma. Bioteh Histochem. 2016;91:96-101.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of home-based neonatal care and management of sepsis on neonatal mortality: Field trial in rural India. Lancet. 1999;35:41955-61.
- [CrossRef] [Google Scholar]
- Comparative estimation of salivary and serum C-reactive protein levels in chronic periodontitis with or without Type II diabetes mellitus: A clinico-biochemical study. SRM J Res Dent Sci. 2017;8:99-104.
- [Google Scholar]
- Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World J Diabetes. 2014;5:730-8.
- [CrossRef] [PubMed] [Google Scholar]
- Selective single-molecule analytical detection of C-reactive protein in saliva with an organic transistor. Anal Bioanal Chem. 2019;411:4899-908.
- [CrossRef] [PubMed] [Google Scholar]
- Jiang HR, Tseng PW. A smartphone-based diffusometric immunoassay for detecting C-reactive protein. Sci Rep. 2019;9:17131.
- [CrossRef] [PubMed] [Google Scholar]
- Current development in microfluidic immunosensing chip. Anal Chim Acta. 2008;611:17-30.
- [CrossRef] [PubMed] [Google Scholar]
- Directed transport of CRP across in vitro models of the blood-saliva barrier strengthens the feasibility of salivary CRP as biomarker for neonatal sepsis. Pharmaceutics. 2021;13:256.
- [CrossRef] [PubMed] [Google Scholar]






