Translate this page into:
Giggles: An indigenous new eye covering device used during neonatal phototherapy
*Corresponding author: Uday Devaskar, Department of Pediatrics, David Geffen School of Medicine at UCLA, Los Angeles, California, USA. udevaskar@mednet.ucla.edu
-
Received: ,
Accepted: ,
How to cite this article: Kalane S, Wagh S, Deshpande M, Kenjale A, Thorat N, Devaskar U. Giggles: An indigenous new eye covering device used during neonatal phototherapy. Karnataka Paediatr J 2021;36(2):98-100.
Abstract
Objectives:
Phototherapy (PT) is commonly used for neonates with indirect hyperbilirubinemia. Dislodgment and inability for spontaneous eye opening are few shortcomings with the traditionally used eye covering devices in neonates while under PT. The study objective was to describe the development of an invention for eye protection in neonatal PT. This invention aims to develop and improvised means that are comfortable for the baby.
Materials and Methods:
The present descriptive study was conducted in level III NICU.
Results:
The invention consists of an eye protector model (Giggles) for use during the neonate’s PT, allowing lesser dislodgements and ophthalmic complications with better esthetics.
Conclusion:
The device is easy to use, and appropriate for neonates, causing no discomfort, and its safety has been proved through radiance tests.
Keywords
Phototherapy
Neonate
Eye covering
Giggles
INTRODUCTION
Phototherapy (PT) is commonly used for more than six decades for treating neonates with hyperbilirubinemia. About 14 million babies worldwide are treated annually with PT. In some patients, PT is needed for more than 5 days. Because of the potential for retinal damage, it is a standard practice to cover both eyes with an eye covering device (ECD).[1] Most of the currently available ECD are tight bands made out of some kind of fabric which prevents the neonate from opening the eye lids [Figure 1]. This tight eye closure is a source of irritation and discomfort for the baby.[2] Forced eye closure can lead to conjunctivitis or keratitis.[3] Displacement of the ECD is a common occurrence needing frequent adjustments and re-applications. Unintentional displacement of these ECD on both the nostrils can lead to life threatening apnea.[4] ECD made out of fabric are not reusable. Finally, they are not aesthetic [Figure 1]. Therefore, a need for developing better ECD was recognized.
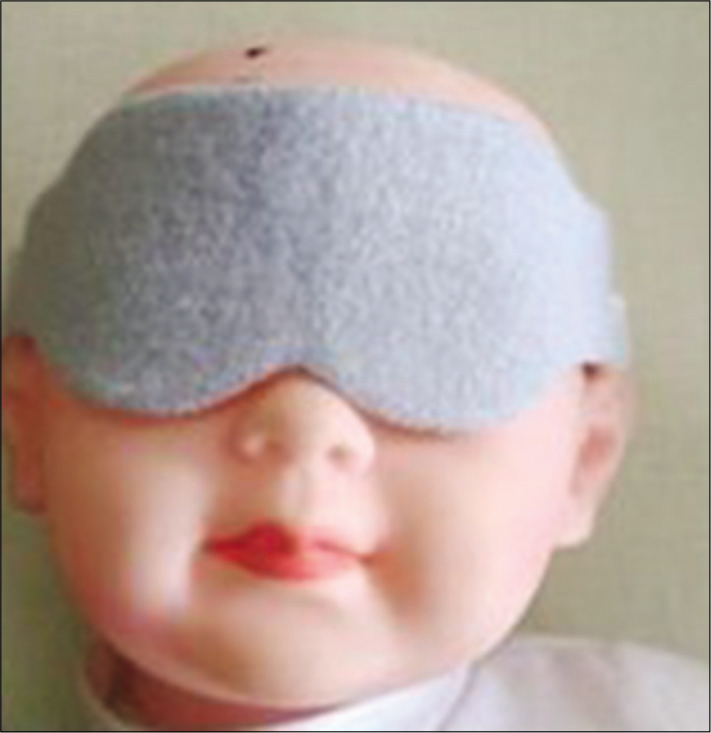
- Traditionally used eye covering.
MATERIALS AND METHODS
The primary design requirements for the new ECD named Giggles were defined: Eliminate forced eye closure, use non-fabric material, it should be soft, light weight, non-allergic, reusable, and easy to apply with an appropriate fitting.
After considering several options including polycarbonate and several elastomers, silicone was considered to be the best material. Silicone, being a non-reactive material, is commonly used in other medical devices. It is soft, flexible, affordable, and easy to wash or sterilize thus suitable for multiple use. The silicone used in making giggles has passed the opacity test, both, before and after exposure to blue light. The approved silicone has passed the test for in vivo bio-compatibility as per ISO-10993.
Head circumference of many neonates was measured before arriving at the appropriate size for the ECD. The dome like structure of the ECD provides space for eye lid opening [Figure 2]. They are flanked with flat rims to give the ECD a comfortable landing around the eyes. The depth of the bubble and a good fit around the head was of major consideration. Finally, it was decided to use multiple slit bands for easy adjustment. The width of the slit had to undergo several alterations to ensure ease of application. A mushroom button, where the band gets fixed to the eye-piece, was positioned close to the eyes to eliminate discomfort when the baby turns his head.

- Giggles eye covering.
RESULTS
An IRB approved randomized control trial comparing giggles (study group) versus commonly used ECD (control) is being conducted at DMH. To date, we have enrolled 52 patients. Preliminary results of the interim analysis are very encouraging favoring use of giggles.
DISCUSSION
Concerns with the use of currently available eve covering devices on newborns receiving phototherapy include the possibility of eye and periorbital skin irritation, corneal abrasion, and infection. Giggles’ one-of-a-kind design should not be associated with these complications. The Giggles ECD is simple and user friendly. The newly developed Giggle ECD we describe provides a convenient and cost effective means of eye protection, and it may be especially useful in busy and understaffed newborn wards and NICU where continuous careful eye-care of infants receiving phototherapy may be impossible.
CONCLUSION
We have developed a new ECD for use during neonatal PT. Giggles is more comfortable allowing the baby to open his eyes, fits better, is safe and hygienic, is easy to apply and clean, reusable, and esthetic. While waiting for the final results of the clinical trial, we believe giggles will be a significant improvement in the ECD used during neonatal PT.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
- [CrossRef] [PubMed] [Google Scholar]
- Abdominal distension in newborn infants on phototherapy the role of eye occlusion. J Pediatr. 1979;94:816-9.
- [CrossRef] [Google Scholar]
- Pseudomonas ophthalmia neonatorum: A case of blindness. Br Med J. 1980;2:440-1.
- [CrossRef] [PubMed] [Google Scholar]
- Airway obstruction by displaced eye mask during phototherapy. Am J Dis Child. 1975;129:1362.
- [CrossRef] [PubMed] [Google Scholar]






