Translate this page into:
Microbiological profile and role of streptokinase in empyema thoraces in children – A retrospective and observational study from a tertiary care teaching hospital from North Karnataka
*Corresponding author: Vinod H. Ratageri, Department of Pediatrics, Karnataka Institute of Medical Sciences, Hubli, Karnataka, India. ratageri@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kanthi AS, Mithra CAG, Ratageri VH. Microbiological profile and role of streptokinase in empyema thoraces in children – A retrospective and observational study from a tertiary care teaching hospital from North Karnataka. Karnataka Paediatr J 2023;38:37-41.
Abstract
Objectives:
Paediatric empyema thoracis causes significant childhood morbidity and mortality over the years. The organisms causing empyema are varying. The role of streptokinase in management is still not clear. Hence, we undertook this study to find the profile of organisms and role of streptokinase in our cases.
Materials and Methods:
A retrospective study was conducted at the Department of Pediatrics, Karnataka Institute of Medical Sciences (KIMS), Hubli from 2017 to 2019. All children aged 2 months–12 years with empyema included. Case sheets were collected from medical records department. The detailed demographic, clinical history, examination findings and treatment received were recorded and entered in a pre-designed performa with a special emphasis on streptokinase usage including indications, dose and outcome. Institution ethical clearance was obtained from Institute Review Board (IRB).
Results:
Thirty-three children fulfilled inclusion criteria during the study period. Mean age was 5.25 ± 1.14 years with male to female ratio of 1.2:1. Most common age group was 1–5 years (39.39%). Clinical manifestations at presentation were fever 100% (33), cough 87.8% (29), dyspnoea 78.78% (26) and intercostal bulge 36.36% (12). Staphylococcus aureus (54.54%) and Streptococcus pneumonia (18.18%) were the most common organisms isolated in pleural fluid. However, blood culture was positive in only one child (non-fermenting Gram-negative bacilli). Fourteen (35%) children received streptokinase. Among those subjects who received streptokinase, 100% were improved as compared to who did not receive (11.5% mortality); however, there was no statistical significance. Although there was decreased duration of hospital stay in streptokinase group (13.79 ± 4.51 vs. 14.73 ± 8.26 days), again this was not statistically significant.
Conclusion:
The most common organism causing empyema was S. aureus. Although use of streptokinase showed better outcome and decreased duration of hospital stay, this was not statistically significant.
Keywords
Empyema thoracis
Bacteriological profile
Antibiotic sensitivity
International classification of diseases
Streptokinase
INTRODUCTION
Empema thoracis in children causes significant morbidity and mortality. It was estimated that 0.6% of childhood pneumonia progress to empyema.[1,2] Empyema thoraces constitutes approximately 5–10% of cases seen by paediatricians in India.[3] Empyema continues to have mortality rate of 5–7%.[4]
Pattern of organisms in the pleural fluid has been varying. Most common causative organisms of empyema are Streptococcus pneumoniae, Staphylococcus aureus, Escherichia coli, Haemophilus influenza, Klebsiella pneumonia, Streptococcus pyogenes and uncommon causative organisms are Mycobacterium tuberculosis and Cryptococcus neoformans.[5,6] In last five decades in developed countries the microbial profile has changed.[7] The prevalence of S. aureus rose and the development of Staphylococcal resistance was observed, but the scenario in developing countries is very different. Even with the use of broad-spectrum antibiotics, prevalence remains high.
Optimal management in children is not clear; however, the mainstay of treatment as per British Thoracic Society (BTS) guidelines is systemic antibiotics and chest tube drainage the role of fibrinolytic agents not yet clear.
Use of streptokinase, urokinase and alteplase has resulted in significant resolution of empyema, with decreased morbidity as well as surgical intervention.[8-10] Contrast to this, some studies have been unable to demonstrate any benefit of intrapleural fibrinolytic therapy in the treatment of empyema in children.[11] Hence, the role of streptokinase is not clear. We present 2-year experience (2017–19) from a tertiary care centre from North Karnataka to provide evidence for the need to optimise the existing management guidelines.
MATERIAL AND METHODS
This was a retrospective study, conducted at Department of Paediatrics, KIMS, Hubli from Jan 2017 to Dec 2019.
Inclusion criteria
All children aged 2 months–12 years with empyema. Empyema was defined by pleural tap showing pus cells under microscopy or purulent exudates on gross examination.[12]
All those fulfilled inclusion criteria were extracted detailed history including predisposing factors, presenting symptoms, signs, all investigations including haemoglobin estimation, total leucocyte count, differential count, C-reactive protein (CRP), blood culture, chest X-ray, ultrasonography (USG) thorax, pleural fluid analysis, specially biochemical analysis, culture and antibiotic sensitivity pattern were recorded in a predesigned performa. Based on pleural fluid analysis, empyema was classified as per modified Light’s criteria.[13] Transthoracic USG was performed in all children.
The details of intercostals drainage, antibiotics used including total duration, response to medical therapy and number of children required surgical therapy were recorded. The details of indication for streptokinase, dose and duration were noted. The association between streptokinase treatment with outcome and duration of hospital stay was studied.
The indication for use of streptokinase was presence of septation on USG chest. Streptokinase was administered through International Classification of Diseases (ICD) tube in a dose of 15,000 units/kg. The dose was diluted to a volume of 50 mL with sterile saline (0.9%) and instilled into the pleural cavity through the ICD tube over 5 min under aseptic precautions.
Statistical analysis
Microsoft excel data sheet was used for data entry and was analysed using SPSS 22 version. Frequencies and proportions represent categorical data. For qualitative data, Chi-square test was used as test of significance. Continuous data were represented as mean and standard deviation. Independent t-test was used as test of significance to identify the mean difference between two quantitative variables.[14]
RESULTS
Total of 33 children fulfilled the inclusion criteria. [Figure 1] shows the flow chart of study children.
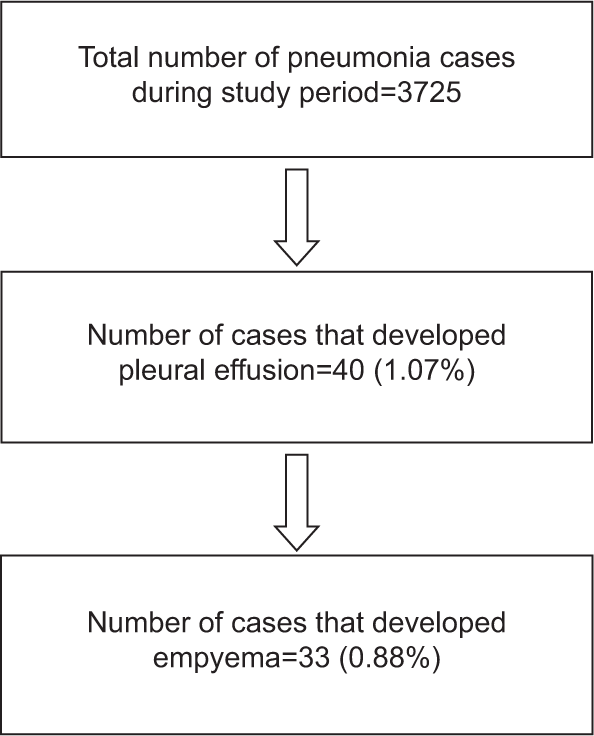
- Flow chart of study
[Table 1] shows baseline characteristics of study children. Mean age was 5.25 ± 1.14 years with Male to female ratio of 1.2:1. Most common age group was 1–5 years (39.39%), of them 54.54% were males and 57.57% belonged to Class III Socio-Econamic Status (SES). The predisposing factors for empyema were severe acute malnutrition (05), nephrotic syndrome (01) and congenital heart disease (01).
| Parameters | Number of children | % |
|---|---|---|
| Age | ||
| <1 year | 5 | 15.15 |
| 1–5 years | 13 | 39.39 |
| 6–10 years | 11 | 33.33 |
| >10 years | 4 | 12.12 |
| Sex | ||
| Female | 15 | 45.45 |
| Male | 18 | 54.54 |
| Socioeconomic status | ||
| Class II | 3 | 9.09 |
| Class III | 19 | 57.57 |
| Class IV | 11 | 33.33 |
Clinical manifestations at presentation of frequency were fever 100% (33), cough 87.8% (29), dyspnoea 78.78% (26), intercostal bulging 36.36% (12), pallor (65%), cyanosis (25%) and pedal oedema (10%).
In the present study, 42.5% had involvement of the right zone of chest, Chest X-ray revealed, along with effusion, 29.5% had consolidation, 20.5% had collapse and 2% had hydropneumothorax.
Mean haemoglobin was 9.54 ± 1.84 g%, mean total leukocyte count was 18055.75 ± 27996.66.67.5%. CRP was positive in 27 (67.5%) children.
In 11 children, organisms were isolated in pleural effusion. Among them, 54.54% (06) were S. aureus, 18.18% (02) S. pneumonia, 9.09% (01) non-fermenting Gram-negative Bacilli, 9.09% (01) Pseudomonas and 9.09% (01) Citrobacter koseri. Only one child (2.5%) had non-fermenting Gram-negative Bacilli grown o blood culture.
[Table 2] shows organisms along with antibiotic sensitivity pattern. S. aureus was sensitive to vancomycin and linazolide while S. pneumonia was sensitive to ceftriaxone. All children were treated initially with third generation cephalosporins and later antibiotics were changed as per antibiotic sensitivity reports. Intercostal drainage was performed in all children of empyema thoraces. Only one child required surgical therapy (decortication). Transthoracic USG was performed in all children. Fourteen children showed septations on USG chest. All fourteen children received streptokinase.
| Organism | Ceftriaxone | Piperacillin-tazobactam | Linezolid | Vancomycin | Amikacin | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| S (%) | R (%) | S (%) | R (%) | S (%) | R (%) | S (%) | R (%) | S (%) | R (%) | |
| Staphylococcus aureus | 2 (33.3) | 4 (66.6) | 1 (16.6) | 5 (83.3) | 5 (83.3) | 1 (16.6) | 4 (66.6) | 2 (33.3) | 3 (50) | 3 (50) |
| Streptococcus pneumonia | 2 (100) | 0 (0) | 1 (50) | 1 (50) | 0 (0) | 2 (100) | 1 (50) | 1 (50) | 2 (100) | 0 (0) |
| Non-fermenting Gram-negative bacilli | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 1 (100) | 0 (0) |
| Pseudomonas | 0 (100) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 1 (100) |
| Citrobacter koseri | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 1 (100) | 0 (0) |
S: Sensitive, R: Resistance
[Table 3] shows the effect of streptokinase in terms of duration and outcome. Among those subjects who received streptokinase, 100% were discharged (improved). Children who did not receive streptokinase had 11.5% mortality. There was no statistical significant association between outcome (mortality) and streptokinase. Mean duration of hospital stay among those who received streptokinase was 13.79 ± 4.51 and who did not receive was 14.73 ± 8.26 days. There was no significant difference in the mean duration of hospital stay with respect to streptokinase usage.
| Parameters | Streptokinase | P-value | |
|---|---|---|---|
| Yes (%) | No (%) | ||
| Outcome | |||
| Discharged | 14 (100.0) | 15 (78.9) | 0.302* |
| AMA | 0 (0.0) | 1 (5.2) | |
| Death | 0 (0.0) | 3 (15.7) | |
| Duration of hospital stay (mean) | 13.79±4.51 | 14.73±8.26 | 0.694 |
DISCUSSION
An increasing incidence of parapneumonic effusion and pleural empyema has been reported in developing countries in recent studies.[15] Paediatric age group is an important risk factor for invasive pneumococcal diseases, with incidence being highest in young children.[16,17] In our study, 1.07% of childhood pneumonia had synpneumonic effusion and 0.88% progressed to empyema.
The bacteriology of pleural infection has been changing in recent years since the introduction of antibiotics in the 1940s.[7] In the present study, among the organisms isolated, most common was S. aureus (54.54%) followed by S. pneumonia (18.18%). S. aureus was the most common organism detected on pleural fluid culture in the study conducted by Lingayat and Wankhade[12] and Ramireddy and Krishna[6] which is similar to our study. Likewise, in a study by Baranwal et al.,[16] S. aureus was the most common organism isolated from pleural fluid (77%). This is related to the emergence of worldwide spread of methicillin-resistant and methicillin susceptible strains. The virulence of S. aureus is due to surface adhesins, toxins, enzymes and superantigens. Panton-Valentine leucocidin is a distinctive virulence factor associated with highly aggressive and fatal form of infection.[18] In a study conducted by Stankey et al.,[19] pleural fluid isolates were S. pneumoniae (48%) followed by group A Streptococcus (27%). S. pneumoniae is an extracellular pathogen characterised by a thick polysaccharide capsule. Specific splenic functions required for immunoglobulin responses to polysaccharide antigens were lacking in children under 2 years. They cannot effectively combat capsulate bacteria and struggle to control pneumococcal colonisation of the nasopharynx, mucosal infections (such as otitis media, sinusitis and pneumonia) and invasive infections (bacteraemia and meningitis).[20]
In our study, 14 children received intrapleural streptokinase. All those children who received streptokinase were improved and discharged. We also observed, there was a decreased duration of hospital stay in streptokinase group, but this was not statistically significant. No adverse effect of streptokinase was noted. Study by Aydoğan et al.,[21] there were no significant differences in terms of clinical outcomes between the two groups. The average length of hospital stay was 19.1 ± 5.5 and 21.9 ± 11.2 days for the drainage and streptokinase groups, respectively. In another study by Altmann et al.,[22] they found no evidence of difference in overall mortality with fibrinolytic versus placebo (Odds ratio 1.16, 95% Confidence interval 0.71–1.91, I2 = 0%) whereas in the study by Ekingen et al.,[23] there was statistically significant difference between two groups according to date of admission (6.8 vs. 10.4 days) and total length of hospital stay (16.6 vs. 22.4 days). Similarly, use of fibrinolytic agents in paediatric empyema resulted in reduced hospital stay, increased chest tube drainage and decrease in intensity of fever in other studies.[24,25] The decreased duration of hospital stay in our cases probably due to streptokinase usage in early stage of disease. In early stage, Streptokinase works by dissolving fibrinous clots and membranes, and prevents fluid sequestration and hence improves drainage.
The strength of our study was having good medical records; however, our study was limited by (i) as it was a retrospective study, (ii) small sample size and (iii) Children who received antibiotics prior to admission were considered that might have hampered culture growth.
CONCLUSION
Paediatric empyema is an important cause of childhood morbidity and mortality. Spectrum of organisms causing empyema is varying in recent years with S. aureus being the most common. Although use of streptokinase showed better outcome and decreased duration of hospital stay, this was not statistically significant.
Declaration of patient consent
Institutional review board (IRB) permission obtained for the study.
Conflicts of interest
Dr. Vinod Ratageri is the Managing Editor of the journal.
Financial support and sponsorship
Nil.
References
- Changing dogmas: History of development in treatment modalities of traumatic pneumothorax, hemothorax, and posttraumatic empyema thoracis. Ann Thorac Surg. 2004;77:372-8.
- [CrossRef] [PubMed] [Google Scholar]
- Parapneumonic empyema deaths during past century, Utah. Emerg Infect Dis. 2009;15:44-8.
- [CrossRef] [PubMed] [Google Scholar]
- Chest tube complications: How well are we training our residents. Can J Surg. 2007;50:450-8.
- [Google Scholar]
- Empyema thoracis: Bacteriological analysis of pleural fluid from the largest chest hospital in Delhi. IOSR J Dent Med Sci. 2013;3:46-51.
- [CrossRef] [Google Scholar]
- A study of pediatric empyema thoracis in a tertiary care hospital. Int J Contemp Med Res. 2016;3:2838-40.
- [Google Scholar]
- Bacteriological profile of pleural fluid among the pediatric population in a tertiary care centre-a retrospective analysis. Int J Health Sci Res. 2015;5:167-74.
- [Google Scholar]
- Intrapleural fibrinolytic treatment of multiloculated postpneumonic pediatric empyemas. Ann Thorac Surg. 2003;76:1849-53.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of intrapleural streptokinase in management of chronic empyemas. J Assoc Physicians India. 2003;51:464-8.
- [Google Scholar]
- Randomised trial of intrapleural urokinase in the treatment of childhood empyema. Thorax. 2002;57:343-7.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized controlled trial of intrapleural streptokinase in empyema thoracis in children. Acta Paediatr. 2004;93:1443-5.
- [CrossRef] [PubMed] [Google Scholar]
- Study of clinical profile, etiological bacterial agents and outcome in pediatric patients of empyema. Indian J Basic Appl Med Res. 2015;4:502-9.
- [Google Scholar]
- Updates in effusion cytology. Surg Pathol Clin. 2018;11:523-44.
- [CrossRef] [PubMed] [Google Scholar]
- Basic biostatistics for post-graduate students. Indian J Pharmacol. 2012;44:435-42.
- [CrossRef] [PubMed] [Google Scholar]
- Etiology of parapneumonic effusion and pleural empyema in children. The role of conventional and molecular microbiological tests. Respir Med. 2016;116:28-33.
- [CrossRef] [PubMed] [Google Scholar]
- Empyema thoracis: A 10-year comparative review of hospitalised children from south Asia. Arch Dis Child. 2003;88:1009-14.
- [CrossRef] [PubMed] [Google Scholar]
- Microbiological diagnosis of empyema in children: Comparative evaluations by culture, polymerase chain reaction, and pneumococcal antigen detection in pleural fluids. Clin Infect Dis. 2006;42:1135-40.
- [CrossRef] [PubMed] [Google Scholar]
- Electronic vaping-induced methicillin-sensitive Staphylococcus aureus pneumonia and empyema. Case Rep Infect Dis. 2021;2021:6651430.
- [CrossRef] [PubMed] [Google Scholar]
- Blood culture and pleural fluid culture yields in pediatric empyema patients: A retrospective review, 1996-2016. Pediatr Infect Dis J. 2018;37:952-4.
- [CrossRef] [PubMed] [Google Scholar]
- Pneumonia in the immunocompetent patient. Br J Radiol. 2010;83:998-1009.
- [CrossRef] [PubMed] [Google Scholar]
- Intrapleural streptokinase treatment in children with empyema. Eur J Pediatr. 2008;167:739-44.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-pleural fibrinolytic therapy versus placebo, or a different fibrinolytic agent, in the treatment of adult parapneumonic effusions and empyema. Cochrane Database Syst Rev. 2019;2019:CD002312.
- [CrossRef] [PubMed] [Google Scholar]
- Fibrinolytic treatment of complicated pediatric thoracic empyemas with intrapleural streptokinase. Eur J Cardiothorac Surg. 2004;26:503-7.
- [CrossRef] [PubMed] [Google Scholar]
- Intrapleural streptokinase for the treatment of childhood empyema. Acta Paediatr Taiwan. 2007;48:251-6.
- [Google Scholar]
- Role of intrapleural streptokinase treatment in children with empyema. Pravara Med Rev. 2016;8:15-9.
- [Google Scholar]






