Translate this page into:
Paraesophageal hernia mimicking pneumatocele in an infant: A diagnostic dilemma
*Corresponding author: Thirunavukkarasu Arun Babu, Department of Pediatrics, All India Institute of Medical Sciences (AIIMS), Mangalagiri, Andhra Pradesh, India. babuarun@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Chirag R, Arun Babu T. Paraesophageal hernia mimicking pneumatocele in an infant: A diagnostic dilemma. Karnataka Paediatr J. doi: 10.25259/KPJ_3_2024
Abstract
A defect in the oesophageal hiatus can result in the herniation of the stomach or other abdominal organs into the thoracic cavity, known as a hiatal hernia. These hernias are uncommon in infants and children, and their symptoms can be vague and non-specific, posing challenges for even experienced clinicians to make a diagnosis. Regardless of the case, surgical intervention is necessary due to the potentially life-threatening complications associated with this condition. We present a rare case of a 2-month-old infant with congenital paraesophageal hernia (PEH) who initially came to our hospital with a diagnosis of pneumonia with pneumatocele. Based on clinical presentation and radiological examinations, the infant was correctly diagnosed with PEH and gastric volvulus. The patient underwent emergency surgical correction and was discharged in a stable condition. It is crucial to have a thorough understanding of the clinical presentation and maintain a high level of suspicion to ensure timely diagnosis and appropriate management in these cases.
Keywords
Children
Pneumonia
Pneumatocele
Paraesophageal hernia
Hiatus hernia
INTRODUCTION
Hiatus hernia is a condition that involves the herniation of the contents of the abdominal cavity, most commonly the stomach, through the diaphragm into the mediastinum.[1] It is more common in adults and uncommon in the paediatric age group, as most cases are caused by acquired age-related laxity of structures supporting the gastroesophageal junction (GEJ). These cases can present with non-specific symptoms such as vomiting, recurrent respiratory system infections or failure to thrive. However, sometimes, they can have an acute clinical presentation with respiratory distress or gastric volvulus.[2,3] As the stomach herniates into the chest, it can be mistaken for cystic lung lesions on radiological images. We present a case of an infant initially misdiagnosed as pneumonia with pneumatocele but later determined to be a para esophageal hernia (PEH).
CASE REPORT
A 2-month-old female child was referred to our hospital with complaints of fever, cough, cold, increased work of breathing and regurgitation of feeds for 12 days. The child had been admitted to a local hospital for seven days, where a chest X-ray and high-resolution computed tomography (HRCT) thorax were performed. The X-ray showed consolidation in the left lung around a pneumatocele, while the HRCT report suggested a small area of consolidation in the right upper lobe of the lung with a pneumatocele, consolidation in the left lower lung and mild left pleural effusion. The child had received intravenous cefotaxime, amikacin and dexamethasone for seven days at the local centre, but the symptoms did not significantly improve, leading to the referral to our centre. The reason for starting on dexamethasone was unclear from the discharge summary.
The child was a full-term, vaginally delivered baby with a birth weight of 2.7 kg. She had a history of 5-day neonatal intensive care unit admission for neonatal jaundice starting from day 2 of life. The child has received appropriate immunisations up to the present. On admission to our hospital, the child appeared active and awake but pale, with a barrel-shaped chest and tachypnoea (respiratory rate: 54/min). Intercostal retractions were prominent, and decreased air entry was noted on the left side of the lung. In addition, an ejection systolic murmur was heard. Due to respiratory distress, the child was started on high-flow nasal cannula therapy, along with empirical antibiotics and intravenous cloxacillin. Initial investigations revealed a haemoglobin level of 10.5 g/dL, total leucocyte count of 20,800, platelet count of 8,900, C-reactive protein level of 0.5 mg/L and thyroid-stimulating hormone level of 1.28 mIU/L. Chest X-ray, taken with an AP view and lateral view using an orogastric tube, showed a large air bubble behind the heart in the left thorax, the absence of a stomach gas bubble and the orogastric tube ending in the left chest [Figure 1a and b]. A 2D echocardiogram suggested a patent foramen ovale with moderate pulmonary arterial hypertension. An ultrasound of the chest revealed a suspicious air bronchogram in the left thorax, while neuro sonography and abdominal ultrasound detected no abnormalities. A repeat HRCT was performed due to unclear images from the previous scans, and it revealed a PEH with herniation of the GEJ, stomach and pylorus into the left hemithorax, causing collapse and consolidatory changes in the lower lobe of the left lung [Figure 2]. The stomach was rotated along its long axis, with the antrum in an anterosuperior location and the fundus in a posteroinferior location. The mediastinum, trachea, bronchi and cardia were shifted to the right side. A contrast study showed contrast material entering the postero-inferior location, followed by the antero-superior location, suggesting gastric volvulus. A diagnosis of the para esophageal type of hiatal hernia with gastric volvulus was made. The child was transferred to paediatric surgery, and surgical repair was performed. She was discharged in a haemodynamically stable condition after ten days.
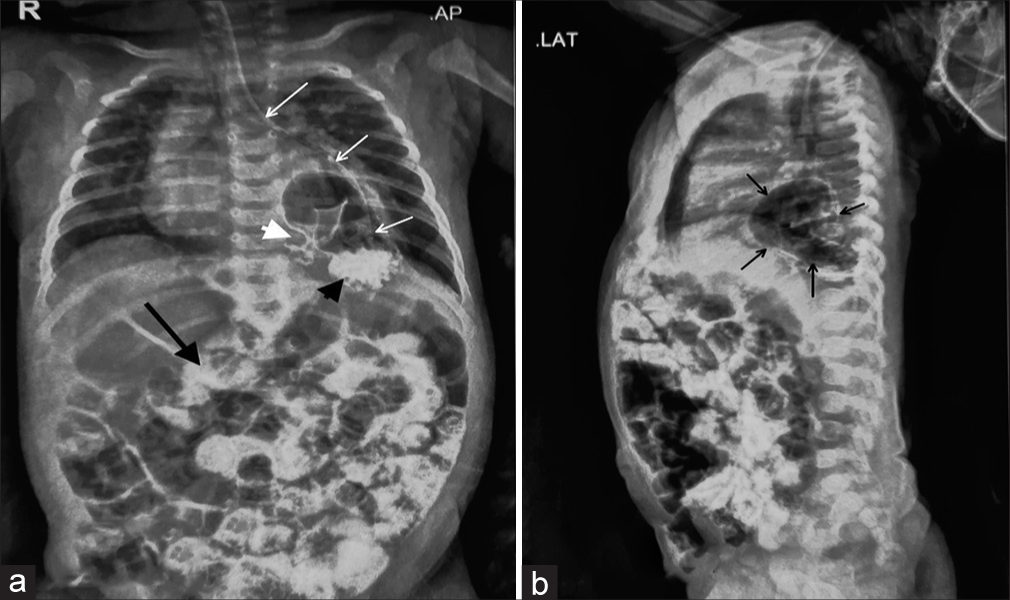
- (a) A chest X-ray AP view post-contrast. Stomach bubble is absent in the abdomen. White arrows trace the orogastric tube, which ends in the left thorax. Contrast delineates the anatomy of the stomach, which is present in the thorax. The black arrowhead points to the contrast material in the stomach fungus. White arrowhead points to the pylorus of the stomach. Contrast can also be seen in the intestine (black arrow). (b) A chest X-ray lateral view post-contrast. The black arrow points to the stomach, which is posterior to the heart shadow. Contrast can also be seen in the intestine.

- High-resolution computed tomography chest showing the stomach (marked by white arrows) behind the heart shadow.
DISCUSSION
Hiatal hernias are of four types: Type I, widely referred to as sliding hernia, occurs when only the gastric cardia protrudes through the oesophageal hiatus and is more common (95% cases of hiatus hernia), and TypesII–IV are known as PEHs and are less common (5% cases of hiatus hernia). Type II occurs when the gastric fundus herniates through the hiatus, but the GEJ remains fixed in position. Type III is a combination of the previous two types in which the GEJ has an abnormal intrathoracic position, and the gastric fundus protrudes into the thoracic cavity. Type IV is the PEH that involves herniation of other abdominal organs such as the colon, small intestine, omentum or spleen.[2,4,5] Due to herniation of the stomach in the thoracic cavity, PEH diagnosis can be confused with cystic lung lesions. In our case, it was confused with pneumatocele. Paraesophageal hernia may present with respiratory distress, especially in Types III and IV[3], as in our case.
The first clue to diagnosis can be chest X-ray AP and lateral view. Chest X-ray may show an absence of gastric bubbles in the stomach and the presence of a well-defined radiolucent shadow in the thorax. The orogastric tube tip may be ending in the thorax. These findings were present in the chest X-ray of our case. Contrast studies are helpful in delineating the content of the herniating organs. Computed tomography can be used to delineate the organ and vascular anatomy. PEH must be differentiated from other cystic lung legions.
Other differential diagnoses include large sliding hiatus hernia, lung abscess, congenital lung cysts, hydatid disease, pericardial cysts, foregut duplication cysts, contained perforations and epiphrenic diverticulum.[6]
Underlying surgical causes should be suspected in neonates or infants with certain scenarios such as persistent or recurrent pneumonia, unilateral pneumonia, absence of response to antibiotics and associated symptoms or a family history of congenital lung disease. An infant or neonate having persistent or recurrent pneumonia with normal immunity, despite appropriate treatment, may raise suspicion for an underlying surgical cause.
Unilateral pneumonia can sometimes be associated with underlying surgical causes. This could include conditions such as lung malformations, lung cysts or bronchial abnormalities.
If an infant or neonate does not show improvement or fails to respond to appropriate antibiotic therapy for pneumonia, it may indicate an underlying surgical cause.
Certain symptoms, such as persistent cough, noisy breathing, respiratory distress, feeding difficulties, failure to thrive or other congenital anomalies that may accompany pneumonia may raise suspicion for underlying surgical causes. A family history of congenital lung or respiratory conditions may increase the likelihood of underlying surgical causes, for example, congenital pulmonary airway malformation. When in doubt, prompt imaging such as chest X-ray, HRCT, bronchoscopy, and barium study will help in the diagnosis of underlying surgical causes.
Potentially life-threatening complications of PEHs include gastric volvulus, bleeding, incarceration, obstruction and gangrene or perforation.[7] Our case presented with complications of gastric volvulus. Surgery is mandatory to treat PEH, even if it is asymptomatic or diagnosed incidentally because if PEH is left untreated, it is prone to the above-cited life-threatening complications.
CONCLUSION
PEH in children is a relatively uncommon entity. It can present with symptoms of recurrent pneumonia. The non-specific symptomatology of PEH can lead to delay or miss diagnosis, leading to diagnostic dilemma and delayed management or mismanagement. Physicians caring for these patients should be aware of such underlying surgical causes predisposing or presenting as pneumonia in children. Prompt diagnosis and surgical correction immediately after the diagnosis are essential to ensure good outcomes for these children.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The diagnosis and management of hiatus hernia. BMJ. 2014;349:g6154.
- [CrossRef] [PubMed] [Google Scholar]
- Paraesophageal hiatus hernia in an 8-month-old infant with organoaxial volvulus of the stomach. Case Rep. 2014;2014:bcr2014204385.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital para esophageal hernia: The Montreal experience. J Pediatr Surg. 2015;50:1462-6.
- [CrossRef] [PubMed] [Google Scholar]
- Hiatal and para esophageal hernia repair in pediatric patients. Semin Pediatr Surg. 2017;26:61-6.
- [CrossRef] [PubMed] [Google Scholar]
- Hiatus Hernia - an overview - ScienceDirect topics. Available from: https://www.sciencedirect.com/topics/medicine-anddentistry/hiatus-hernia [Last accessed on 2023 May 22]
- [Google Scholar]
- Primary para esophageal hernia in children. J Pediatr Surg. 2006;41:1588-93.
- [CrossRef] [PubMed] [Google Scholar]
- A surgical emergency due to an incarcerated para esophageal hernia. Am J Emerg Med. 2009;27:134.e1-3.
- [CrossRef] [PubMed] [Google Scholar]






