Translate this page into:
A nutritional Vitamin B12 deficiency (infantile tremor syndrome) presenting as hemiconvulsion hemiplegia epilepsy syndrome
*Corresponding author: Vykuntaraju K. Gowda, Department of Pediatric Neurology, Indira Gandhi Institute of Child Health, Bengaluru, Karnataka, India. drknvraju08@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gowda VK, Challa VR, Srinivasan VM, Narayana Vamyanmane D. A nutritional Vitamin B12 deficiency (infantile tremor syndrome) presenting as hemiconvulsion hemiplegia epilepsy syndrome. Karnataka Paediatr J. 2024;39:60-4. doi: 10.25259/KPJ_58_2023
Abstract
Hemiplegia Hemiconvulsion Epilepsy syndrome (HHE syndrome) is a condition characterized by the acute onset of unilateral seizures, which progress to cortical atrophy, hemiplegia and later develop epilepsy over weeks to years. Various aetiologies include infectious causes, especially meningitis, encephalitis, trauma, vascular insults, and sometimes idiopathic. Here, we present a 13-month-old female child, first in birth order, born to a non-consanguineous marriage, who presented with mild global developmental delay with acute onset right focal seizures followed by right-sided weakness and drug-resistant epilepsy. The child was found to have clinical and biochemical features of vitamin B12 deficiency with right-sided weakness. MRI of the brain initially showed edema of the left cerebral hemisphere and later atrophy on the left side. The electroencephalogram showed low voltage activity over the left cerebral hemisphere. Post-treatment with cobalamin, improvement in development was noted, with partial improvement in weakness and persistent epilepsy. In the current report, we highlight Hemiplegia Hemiconvulsion Epilepsy (HHE) syndrome on the background of nutritional vitamin B12 deficiency.
Keywords
Hemiconvulsion hemiplegia epilepsy syndrome
Nutritional Vitamin B12
Infantile tremor syndrome
Cerebral atrophy
Hemiplegia
INTRODUCTION
Hemiplegia Hemiconvulsion Epilepsy (HHE) syndrome is a condition in which the patient develops unilateral seizures followed by ipsilateral cerebral oedema progressing to cortical atrophy which manifests as hemiplegia and finally develops epilepsy over weeks to months of varying frequency.[1] The various aetiologies include hypercoagulable states such as protein C/S deficiency, factor V deficiency, metabolic disorders like L2 hydroxy glutaric aciduria, infectious agents like influenza, human immunodeficiency virus, hypoxia, ischemia, toxic agents like theophylline and sometimes unknown cause.[2-4] The main pathophysiology behind the syndrome involves any aetiology which causes prolonged seizures that lead to histotoxic damage of the parenchyma of the brain.[5] There is a complex interplay between genetic, infectious and environmental agents to precipitate HHE syndrome.[4] Here, we present an unusual case of HHE syndrome with nutritional Vitamin B12 deficiency without any apparent aetiology.
CASE REPORT
A 13-month-old female child born to non-consanguineous marriage presented with loose stools, vomiting and fever since the past 5 days followed by right focal status epilepticus. On day 2 post seizures, the right-sided upper and lower limb weakness was noted, in the background of normal birth history and mild developmental delay. At 13 months of age, the child could sit but could not walk, was able to respond to name inconsistently and could not speak any meaningful words. Child was on predominant breast feeds with the mother’s diet primary vegetarian and initiated on complementary feeds by 8 months. There was a history of vomiting episodes with decreased acceptance of feeds. On examination, Weight of 7.5 kg (−1 to −2SD), height of 76 cm (0 to −1SD), head circumference of 44 cm (−1 to −2SD), mid-arm circumference of 11.5 cm was noted. There was hypopigmented hair and knuckle hyperpigmentation noted [Figure 1]. Neurological examination revealed Glasgow coma scale: 9/15, right-sided upper motor facial palsy, hypotonia on the right side with power of 2/5 on Medical Research Council (MRC) grade [Figure 1a] and extensor plantar on the right side along with tremors.

- Clinical photograph of child before discharge (a) shows hypopigmented hair, pallor, knuckle hyperpigmentation, mild right-sided facial weakness with the right upper weakness at the time of discharge – 12 months of age. During follow-up (b) shows weakness of the right upper limb with wasting and increased tone during follow-up at 4 years of age.
Investigations showed haemoglobin of 8.6 g/dL, mean corpuscular volume (MCV) of 106.7, with normal total leukocyte and platelet counts and megaloblastic anaemia in the peripheral smear with low Vitamin B12 level of 120 pg/mL (normal 180–300 pg/mL) and increased levels of homocysteine of 79.83 μmol/L (normal 3–15 μmol/L). The renal function tests, tandem mass spectrometry for inborn errors of metabolism, cerebrospinal fluid (CSF) analysis, fasting ammonia, lactate and arterial blood gas done were normal. Electroencephalogram (EEG) showed asymmetric background activity with low voltage over the left cerebral hemisphere. Magnetic resonance imaging (MRI) brain shows diffuse oedema of the left hemisphere, corpus callosum, left internal capsule and bilateral globus pallidus regions appearing hyperintense on axial diffusion-weighted imaging [Figure 2a and b] and hyperintense on fluid-attenuated inversion recovery (FLAIR) [Figure 2c] sequences. Magnetic resonance angiography [Figure 3a] shows no obstruction or narrowing of vessels. Magnetic resonance venogram [Figure 3b] shows a reduced number of vessels and flow in the left cerebral hemisphere. Mother investigations revealed haemoglobin of 9.5 g/dL, MCV of 101.7, with normal total leukocyte and platelet counts and megaloblastic anaemia in the peripheral smear with low Vitamin B12 level of 145 pg/mL (normal 180–300 pg/mL) and increased levels of homocysteine of 49.83 μmol/L (normal 3–15 μmol/L). The whole exome sequencing and mitochondrial genome sequencing did not reveal any pathogenic variant.
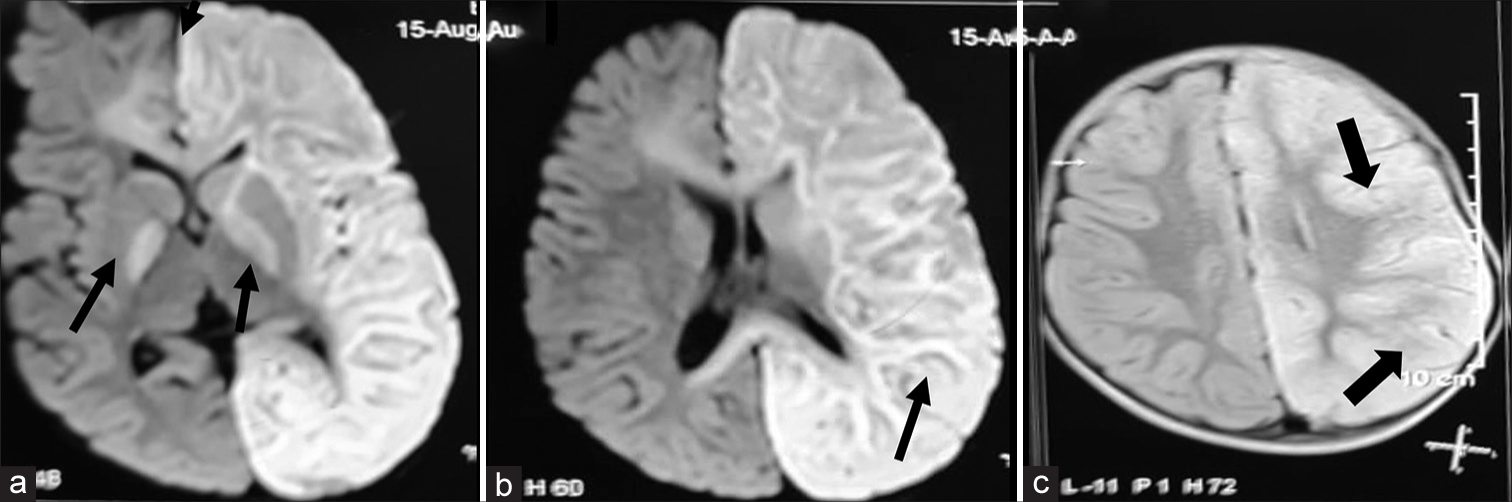
- (a and b) Magnetic resonance imaging brain shows diffuse oedema of the left hemisphere, corpus callosum, left internal capsule and bilateral globus pallidus regions appearing hyperintense (black arrows) on axial diffusion-weighted imaging and hyperintense (black arrows) on fluid-attenuated inversion recovery (c) sequences on 7th day at 13 months of age when admitted to the hospital (black arrows).
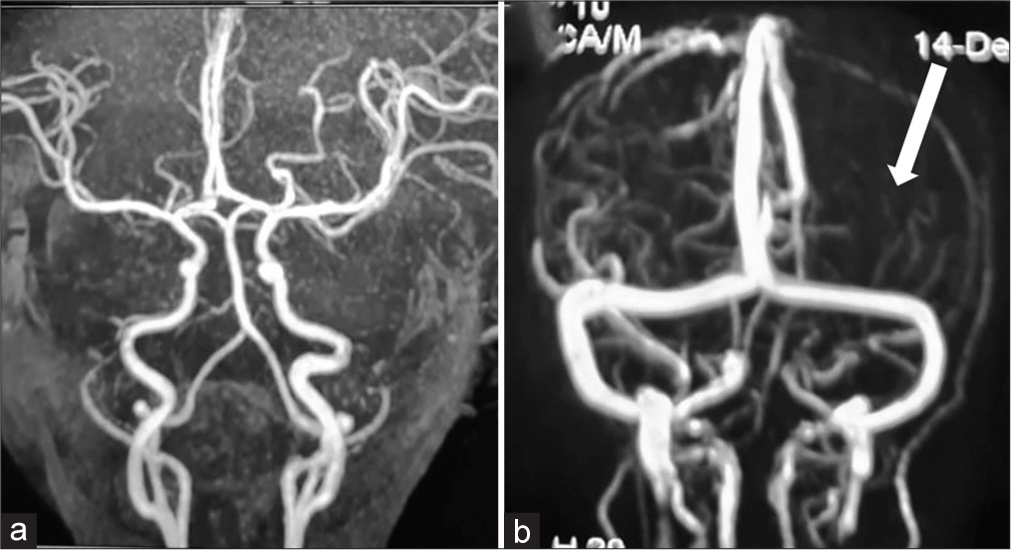
- Magnetic resonance angiography (a) shows no obstruction or narrowing of vessels. Magnetic resonance venogram (b) shows reduced number of vessels and flow in the left cerebral hemisphere (thick white arrow) on the 7th day at 13 months of age when admitted to the hospital.
A diagnosis of HHE syndrome due to nutritional Vitamin B12 deficiency was made. Child was treated with injectable cobalamin for 14 days, folic acid and other supplements with marked improvement in development on follow-up. After 2 weeks of treatment, investigations revealed haemoglobin of 9.6 g/dL, Vitamin B12 level of 859 pg/mL (normal 180–300 pg/mL) and homocysteine of 9.83 μmol/L (normal 3–15 μmol/L). The weakness of the right side improved partially and developed stiffness of the right upper limb [Figure 1b] and right lower limb. Repeat MRI brain at 4 years showed left hemisphere atrophy [Figure 4a] with no restricted diffusion. Diffuse gliotic areas in the left cerebral subcortical white matter appearing hyperintense on axial FLAIR [Figure 4b] and hypointense on T1-weighted sequences [Figure 4c]. EEG of child done at 4 year of age showed low voltage over the left cerebral hemisphere [Figure 5]. Child was on regular follow-up with dietary advice of Vitamin B12 rich diet without any Vitamin B12 supplements and serum Vitamin B12 and homocysteine levels were within normal limits over the 4 years follow-up period.
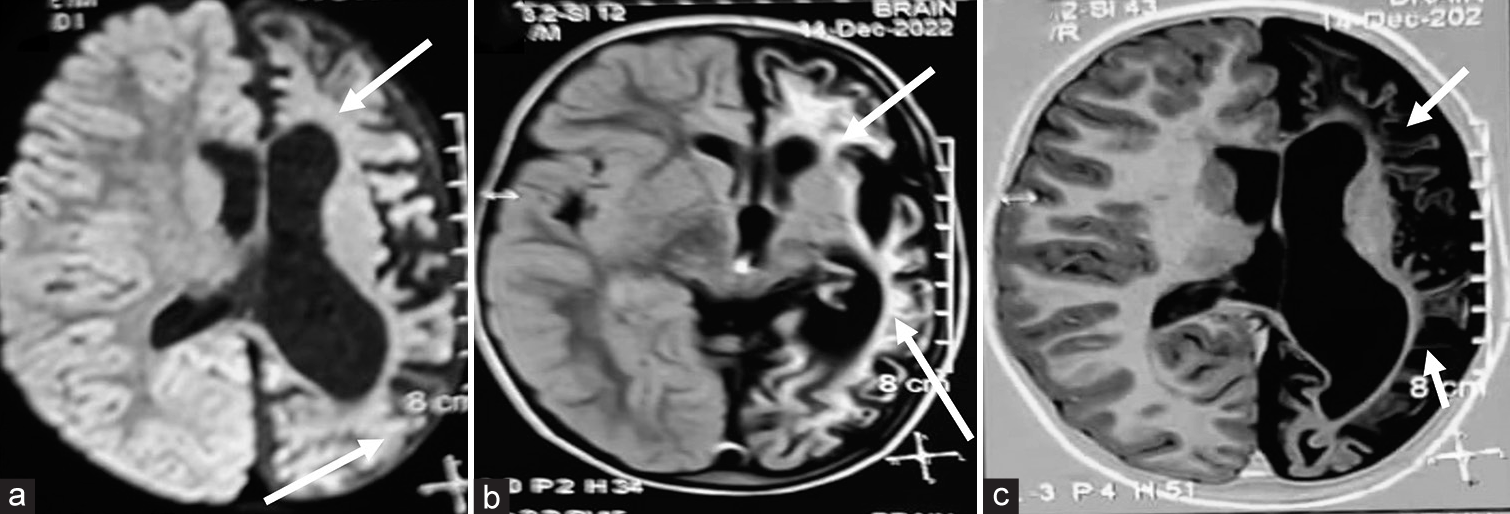
- (a-c) Repeat magnetic resonance imaging brain at 4 years. Axial diffusion weighted sequence showing left hemisphere atrophy (thick white arrows) with no restricted diffusion. Diffuse gliotic areas in the left cerebral subcortical white matter appearing hyperintense on axial fluid-attenuated inversion recovery and hypointense (thin white arrows) on T1-weighted sequences.
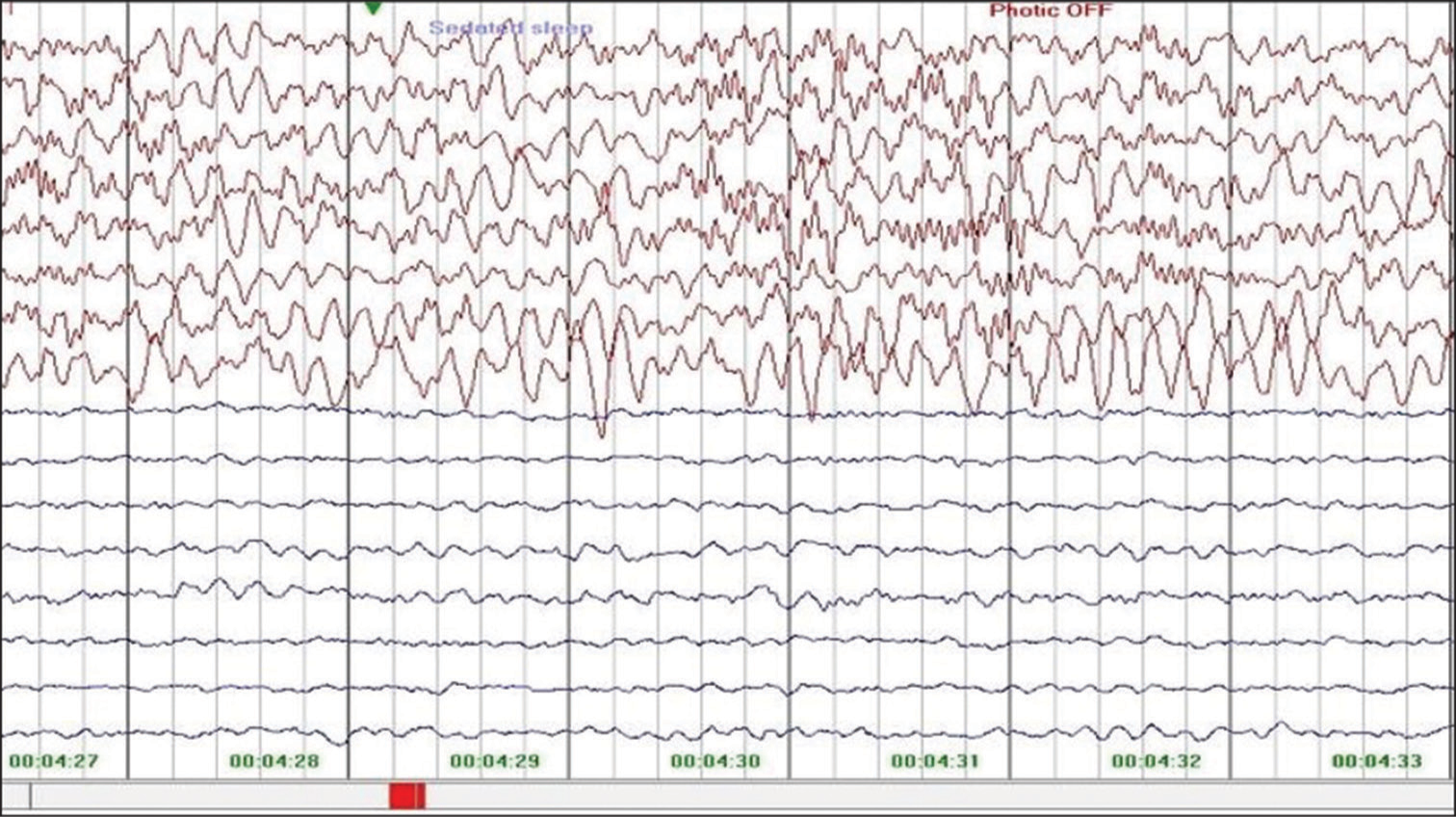
- Electroencephalogram of child done at 4 years of age showing low voltage over the left cerebral hemisphere during follow-up at 4 years of age.
DISCUSSION
The differential diagnosis considered were HHE syndrome, Rasmussen’s encephalitis, infectious aetiologies such as meningitis and encephalitis and cerebrovascular accidents such as cerebral venous thrombosis secondary to acute gastroenteritis induced dehydration. CSF analysis and MRA done were normal, ruling out neuroinfections and stroke. The patient had clinical features and biochemical evidence of Vitamin B12 deficiency; hence, nutritional Vitamin B12 deficiency was considered as a potential aetiology. Rasmussen’s encephalitis presents with insidious onset of focal seizures such as epilepsia partialis continua, followed by ipsilateral weakness over a period of months and years with MRI of the brain showing atrophy involving caudate nucleus, and later temporal lobe. HEE children present with focal status followed by weakness maximum at onset later other type of seizures and MRI initially shows contralateral hemispheric oedema involving all lobes followed by hemispheric atrophy of all lobes; hence, our case features are suggestive of HHE, rather than Rasmussen’s encephalitis.
Methyl cobalamin is an active form of Vitamin B12 required for maintenance of myelin in the nervous system and deficiency usually leads to myelin disturbances. The neurons of the cerebral cortex with myelin disruption may be prone to glutamate excitotoxicity which could be a reason for seizures.[6] Furthermore, in the absence of cobalamin, homocysteine cannot be converted to methionine which leads to the elevation of the former. Under experimental conditions, high doses of homocysteine have induced convulsive status in animals.[7] In the current case, the patient had elevated homocysteine, low Vitamin B12 which could be the cause for prolonged seizures and the possible aetiology of Vitamin B12 deficiency seems to be nutritional in origin.
The usual course of HHE syndrome is prolonged unilateral seizures of clonic type which leads to brain oedema in acute stages. Initially, the syndrome has only HH component with E component of the disease developing over weeks to months. The neuroimaging shows cerebral atrophy after resolution of oedema.[8] The outcomes are variable with temporal lobe epilepsy in idiopathic and generalised seizures in symptomatic type after a long symptom free period of weeks to months. Many patients have variable degrees of motor, cognitive and language impairment.[9]
The current case showed clinical and laboratory features of nutritional Vitamin B12 deficiency, features of HHE syndrome as focal status seizures followed by ipsilateral weakness and later epilepsy. We ruled out other causes for weakness based on MRI and MRA of the brain, metabolic and genetic causes based on negative metabolic workup and genetic workup. Myers et al. reported a 4-year-old female with Cobalamin C disease due to homozygous MMACHC mutation who presented with features of HHE syndrome.[10] However, there are no reports which link nutritional Vitamin B12 deficiency to HHE syndrome. This is a rare association.
CONCLUSION
Nutritional Vitamin B12 deficiency should be considered as the risk factor of HHE syndrome as it would have an impact on the treatment and prognosis and needs further studying.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Hemiconvulsionhemiplegia-epilepsy syndrome: Clinical course and neuroradiological features in a 20-month-old girl. BMJ Case Rep. 2014;2014:bcr2013203482.
- [CrossRef] [PubMed] [Google Scholar]
- Hemiconvulsion, hemiplegia, epilepsy syndrome and inherited protein S deficiency. Indian J Pediatr. 2006;73:157-9.
- [CrossRef] [PubMed] [Google Scholar]
- Association between factor V Leiden mutation and the hemiconvulsion, hemiplegia, and epilepsy syndrome: Report of two cases. J Child Neurol. 2002;17:713-7.
- [CrossRef] [PubMed] [Google Scholar]
- Acute encephalopathy associated with influenza and other viral infections. Acta Neurol Scand Suppl. 2007;186:45-56.
- [CrossRef] [Google Scholar]
- Hemiconvulsion-hemiplegia-epilepsy syndrome. A clinical, electroencephalographic and neuroradiological study. Childs Nerv Syst. 1997;13:257-63.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effects of vitamin B12 analog, methyl cobalamin, against glutamate cytotoxicity in cultured cortical neurons. Eur J Pharmacol. 1993;241:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Seizures induced by homocysteine in rats during ontogenesis. Epilepsia. 1995;36:750-6.
- [CrossRef] [PubMed] [Google Scholar]
- Hemiconvulsion-hemiplegia-epilepsy syndrome: Early magnetic resonance imaging findings and neuroradiological follow-up. Brain Dev. 2007;29:109-11.
- [CrossRef] [PubMed] [Google Scholar]
- Teaching NEUROIMAGE: Hemiconvulsion-hemiplegia-epilepsy syndrome: Sequential MRI follow-up. Neurology. 2008;71:e28.
- [CrossRef] [PubMed] [Google Scholar]
- Hemiconvulsion-hemiplegiaepilepsy in a girl with cobalamin C deficiency. Epileptic Disord. 2018;20:545-50.
- [CrossRef] [PubMed] [Google Scholar]







