Translate this page into:
Clinico-epidemiological profile of children with atopic dermatitis
*Corresponding author: Sharang Gupta, Department of Dermatology, Government Medical College, Patiala, Punjab, India. drsharanggupta97@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sidhu RD, Kaur L, Chopra D, Gupta S, Rajenesh A, Sidhu AS. Clinico-epidemiological profile of children with atopic dermatitis. Karnataka Paediatr J. doi: 10.25259/KPJ_42_2024
Abstract
Objectives:
This study was conducted to assess clinico-epidemiological features of atopic dermatitis (AD) in children and evaluate disease severity using the SCORing atopic dermatitis index (SCORAD Index).
Material and Methods:
This was a hospital-based cross-sectional study carried out at the department of Dermatology at a tertiary care centre in North India over a period of 1 year. This study enrolled children aged between 3 months and 16 years of age clinically diagnosed with AD using Hanifin and Rajka criteria (HRC).
Results:
The prevalence of AD was found to be 8.12%. Age of presentation ranged from 4 months to 16 years of age, with a mean age of 4.18 ± 3.88 years. 42% of patients had personal and 52.50% of patients had a family history of atopy. We report pruritus as a major presenting symptom seen in 100% of children with AD. Typical morphology and distribution were observed in 97.50% of cases, with the face being the most commonly affected site in infants (95.08%) and flexural involvement more common in children (77.70%). Infants had acute eczema (63.93%) and children with AD mostly presented with chronic eczematous lesions (58.27%). The most common minor feature of HRC observed was xerosis, seen in 94% of patients. Amongst atypical features, scalp scaling was the most common feature seen in 17%. SCORAD Index grading was mild in 49.50% of children with AD, moderate in 40.50% and severe grading was seen only in 10% of children.
Conclusion:
Clinical features, prevalence and severity of AD are affected by various geographical, environmental and local factors such as lifestyle, clothing and eating/dietary factors.
Keywords
Atopic dermatitis
Eczema
SCORAD
INTRODUCTION
Wise and Sulzberger, in 1933, first used the term atopic dermatitis (AD).[1] It is a chronic inflammatory, intensely pruritic disease of the skin which has frequent exacerbations and remissions and a personal or family history of ‘atopic diathesis’ in most of the patients.[2] It manifests mostly during infancy or childhood but can also present in adulthood.[3]
AD has multifactorial aetiology with a complex interplay of various internal and external factors, including genetic susceptibility leading to epidermal barrier dysfunction, defects in immune responses (innate and adaptive) and heightened immunological reactions to environmental allergens and microbial antigens.
There is geographical variability in the prevalence of AD, which has been attributed to various environmental factors, including rapid urbanisation, dietary factors, the climate of the region, infections and aeroallergens.[4] Considering the prevalence of AD in India, most of the Indian centres which participated in the International Study of Asthma and Allergies in Childhood study reported the prevalence of AD in age groups 6–7 years and 13–14 years to be <5%. However, there is a dearth of clinico-epidemiological data on AD from the northern part of India in the existing literature. Hence, this study was conducted to assess clinicoepidemiological features of AD in children and evaluate disease severity using the Scoring Severity of the Atopic Dermatitis Index (SCORAD Index).
Aims and objectives
The present study was conducted to assess clinicoepidemiological features of AD in children and evaluate disease severity using the SCORing Atopic Dermatitis Index (SCORAD Index).
MATERIAL AND METHODS
This was a hospital-based cross-sectional study conducted at the Department of Dermatology at a tertiary care centre in North India over a period of 12 months after approval of the institutional ethics committee.
Sample size determination
The sample size was calculated using the following formula[5]
n = Za/22 *p*(1–p)/d2
n = is the desired sample size
Z = Standard normal deviation usually set at 1.96, corresponding to 95% confidence level
Z is the critical value of the normal distribution at a/2 (e.g. for a confidence level of 95%, a is 0.05 and the critical value is 1.96)
* is the multiplication symbol in the above equation d = is the margin of error or degree of accuracy desired, usually set at 5% or 0.05
p = the proportion in the target population estimated to have the particular disease. Expected frequency of AD in the desired age group in the study based on a 2-year average as per hospital record = 8.5% (0.85)
Finite population correction was applied to the sample size formula.
n = Z2 p q/d2
q = 1.0–p (1.0–0.85) = 0.15
In this study:
Z = 1.96, p = 8.5% (0.85), q = 1.0–0.85 = 0.15, d = 0.05
n = 1.96 × 1.96 × 0.85 × 0.15/0.05 × 0.05 = 195 was the minimum sample size. Two hundred patients were recruited for the study.
Inclusion criteria
Cases of AD diagnosed clinically with Hanifin and Rajka criteria (HRC) between age group of 3 months–16 years.
Exclusion criteria
Paediatric patients in the same age group with other dermatoses such as scabies, childhood psoriasis, irritant and allergic contact dermatitis, seborrhoeic dermatitis, immunodeficiency syndromes and metabolic diseases
Parents/guardians are not willing to take part in the study.
Written consent from parents/guardians was taken. The severity of AD was studied using the SCORAD Index.[6,7]
Statistical analysis
Data were analysed using Microsoft Excel Office software 2019 version 19.1 and statistical analysis was done using the Statistical Package for the Social Sciences for Windows version 22. Continuous variables (i.e., age of onset and SCORAD Index values) were expressed as mean ± standard deviation (SD) values and range. An appropriate statistical test of comparison was applied. Continuous variables were analysed with a t-test. Categorical variables (i.e., gender, geographical distribution severity, history of atopy, major and minor features) were expressed as numbers, frequency and percentages (%); the Chi-square test was used to compare categorical data. All statistical tests were performed at a significance level of P < 0.05.
RESULTS
2462 cases aged 3 months–16 years attended the outpatient department of Dermatology during the study period of 1 year, out of which 200 cases were clinically diagnosed as AD using HRC, contributing to a hospital prevalence of 8.12%.
Out of 200 patients, 89 (44.50%) were females and 111 (55.50%) were males, with a male: female ratio of 1.25:1. Study sample was divided into 5 age groups: Age group 3 months–< 1 year, 1–5 years, 6–10 years, 11–15 years and >15 years. Table 1 shows the distribution of children with AD according to age and gender.
| Age Group (Years) | Female | Male | Total | |||
|---|---|---|---|---|---|---|
| Patients | (%) | Patients | (%) | Patients | (%) | |
| 3 Months–<1 Year | 16 | 17.98 | 28 | 25.23 | 44 | 22 |
| 1–5 | 48 | 53.93 | 56 | 50.45 | 104 | 52 |
| 6–10 | 12 | 13.48 | 22 | 19.82 | 34 | 17 |
| 11–15 | 11 | 12.36 | 5 | 4.50 | 16 | 8 |
| >15 | 2 | 2.25 | 0 | 0 | 2 | 1 |
| Total | 89 | 100 | 111 | 100 | 200 | 100 |
AD: Atopic dermatitis
Demographic and socioeconomic status is shown in Figures 1 and 2, respectively. Out of 200 patients in the study sample, 62.50% (125) children had disease onset by 1 year and only 9% (18) had onset after 5 years of age. 91% (182) of children had onset of disease under 5 years of age. The mean ± SD age at onset was 1.98 ± 2.24 years in the study group. The minimum to the maximum age of onset was 4 months to 10 years, respectively.
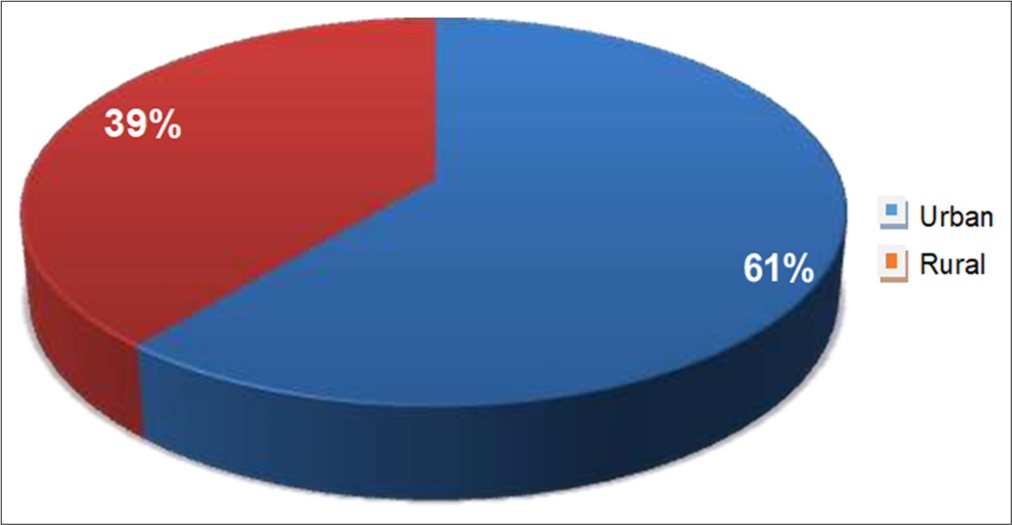
- Geographical distribution of patients with atopic dermatitis.
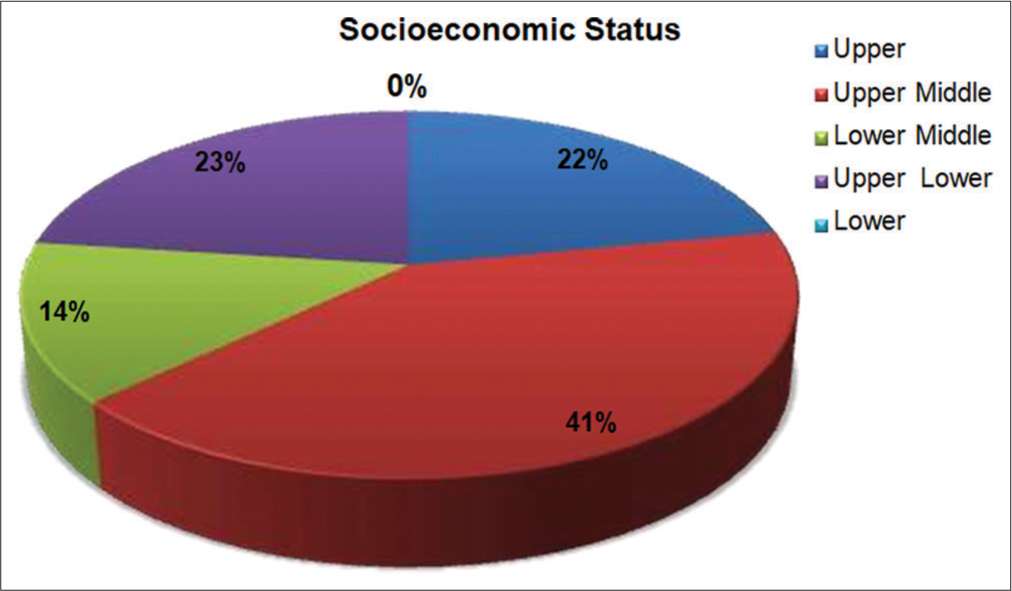
- Socioeconomic status of children with atopic dermatitis.
The frequency of history of atopy in patients of AD is shown in Table 2. Figure 3 shows the frequency of factors influencing the course of disease in children with AD. It was noted in the study sample that disease aggravation was more prevalent in winter.
| History of atopy | Patients (n) | Percentage |
|---|---|---|
| Personal history | 84 | 42 |
| Allergic rhinitis | 62 | 73.81 |
| Bronchial asthma | 22 | 26.19 |
| Family history | 105 | 52.50 |
| Allergic rhinitis | 82 | 78.09 |
| Bronchial asthma | 12 | 11.43 |
| Atopic dermatitis | 11 | 10.48 |
| Both personal and family history of atopy | 28 | 14 |
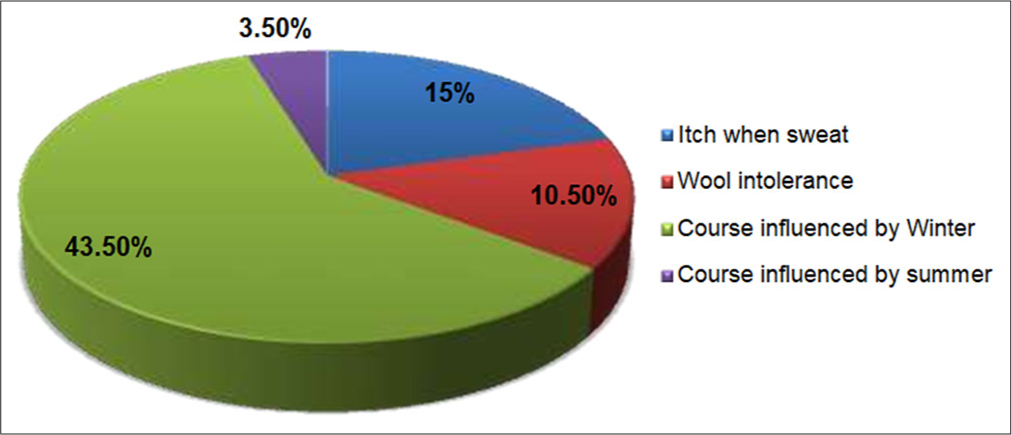
- Frequency of factors influencing the course of the disease.
100% of patients had pruritus as a presenting symptom, followed by typical morphology and distribution of lesions in 97.50% (195) and a history of chronic/relapsing dermatitis in 75% (150) children.
The distribution of minor clinical features of HRC is shown in Table 3. Amongst atypical features, scalp scaling was the most common, followed by retro-auricular fissuring [Figure 4]. Infants had acute eczema with the face being affected commonly, whereas older children had chronic eczematous lesions with flexural involvement.
| Minor criteria | Patient | Percentage |
|---|---|---|
| Xerosis | 188 | 94 |
| Hand and foot eczema | 82 | 41 |
| Facial erythema | 81 | 40.50 |
| Cutaneous infection | 61 | 30.50 |
| Dennie morgan fold | 43 | 21.50 |
| Pityriasis alba | 32 | 16 |
| Cheilitis | 19 | 9.50 |
| Perifollicular accentuation | 19 | 9.50 |
| Periorbital darkening | 18 | 9 |
| Anterior neck sign | 15 | 7.50 |
| Facial pallor | 12 | 6 |
| Ichthyosis | 7 | 3.50 |
| Hyperlinear palms | 4 | 2 |
| Keratosis pilaris | 2 | 1 |
HRC: Hanifin and Rajka criteria

- Frequency of atypical clinical features in children with atopic dermatitis.
The severity of the disease was mild in 49.50% (99) children, moderate disease in 40.50% (81), and only 10% (20) children had severe disease. The mean SCORAD Index was 27.57 ± 14.91. Mean SCORAD Index in mild, moderate and severe disease was 15.25 ± 5.09, 35.39 ± 7.04 and 56.88 ± 5.39, respectively. SCORAD Index range was 4.5 – 73.
Figure 5 shows the age-wise severity grading of children with AD. Mean SCORAD Index in 3 months–<1, 1–5, 6–10, 11–15, >15 years was 0.62 ± 0.21, 2.93 ± 1.33, 7.65 ± 1.38, 13.25 ± 1.39, 16.00 ± 0.00, respectively. Children in whom disease onset was under 5 years of age were statistically more in number in all three grades of disease severity (P = 0.001). All children (20) with severe SCORAD Index grading had disease onset under 5 years of age and it was found to be statistically significant P = 0.001.
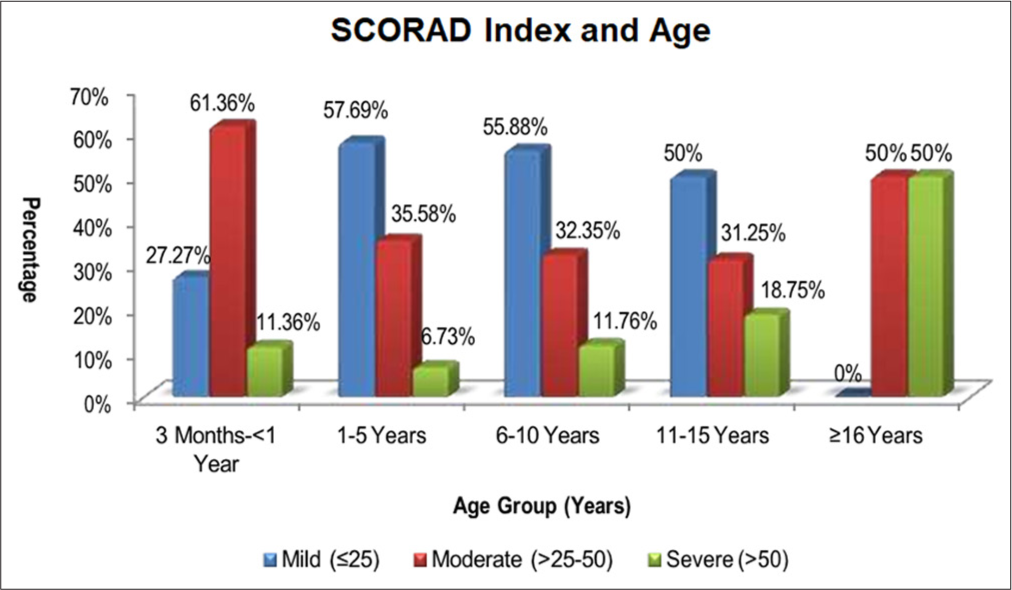
- Age-wise severity grading of children with atopic dermatitis.
Retro-auricular fissuring, infra-auricular fissuring and scalp scaling were found to be more significantly associated with the severity of the disease than others, with P = 0.001, 0.010 and 0.016, respectively [Table 4].
DISCUSSION
Hospital prevalence of AD was found to be 8.12%. The study done by Singh and Yadav[8] (Jaipur) Sehgal et al.[9] (Delhi) and Sarkar and Kanwar[10] (North India) have reported a prevalence of 8.52%, 0.24% and 29.9%, respectively. Such variation in AD prevalence is attributed to environmental and socioeconomic factors. The prevalence of AD in India is a rising trend, which is attributed to urbanisation and increased environmental pollution, hence exposure to allergens.[10]
M: F ratio in this study came out to be 1.25:1. Our results were comparable with a study conducted by Dhar et al.[11] (2002). One of the reasons for male preponderance seen in Indian hospital-based epidemiological studies is that there is more treatment-seeking behaviour for males compared to females.
Seventy-four percent of children with AD were in the age group of 3 months–5 years. Narayan et al.[12] (2019) and Swamy et al.[13] (2019) reported a similar age distribution with 78% and 74% of children with AD below 5 years of age. The mean age of presentation was 4.18 ± 3.88 years, which is almost comparable with that of the study by Parthasarathy et al.[14] (2019), who reported the mean age of presentation as 4.6 ± 3.9 years. The mean age of onset was 1.98 ± 2.24 years. The mean age of onset corroborates with other Indian studies done by Narayan et al.[12] (2019) 2.13 ± 2.10 years and Upendra et al.[15] (2018) 2.14 ± 0.52 years.
62.50% of children had onset of disease by the age of 1 year, and only 9% developed AD after 5 years of age. Sendrasoa et al.[16] (2020) reported an early age of onset of 5 years in 69.5% of children. In North India, Sarkar and Kanwar[10] (2004) reported that 55.2% of patients had the disease by 1 year of age and 5.6% developed AD after 6 years of age.
In this study, 61% of AD patients were from urban areas and only 39% from rural areas. A study done by Sarkar and Kanwar (2004) and Thakur et al. (2013) observed similar findings.[10,17] AD is more prevalent in urbanised areas, as people in these settings are more exposed to exacerbating factors such as chemicals, aeroallergens and environmental pollution.[18]
The majority of children with AD in this study were from middle socio-economic class 55% followed by upper 23% and lower 22%. These findings revealed almost comparable results to other Indian studies by Swamy et al.[13] (2019). Overall, upper and middle-class populations were more affected by AD due to different food habits, living standards, hygiene and psychological stress.[19]
In our study, 42% of patients had either one or more of 3 manifestations of atopy-allergic rhinitis, bronchial asthma and conjunctivitis, whereas 52.50% of patients had a history of atopy in their families (up to three generations). Fourteen percent had both personal and family history of atopy. Personal and family history of atopy has variable results in different studies, with personal history ranging from 18.18% to 59.25%[11,12,20-22] and family history having variability of 37.03% to 90.67%.[11,12,15,20-22]
| Atypical clinical features | Patients | Mild (n=99) | Moderate (n=81) | Severe (n=20) | X2 | P-value | |||
|---|---|---|---|---|---|---|---|---|---|
| n | Percentage | n | Percentage | n | Percentage | ||||
| Scalp scaling | 34 | 5 | 5.05 | 17 | 21.08 | 12 | 60 | 5.79 | 0.016 |
| Retro auricular fissuring | 20 | 0 | 0 | 5 | 6.17 | 15 | 75 | 20.91 | 0.001 |
| Eyelid eczema | 15 | 2 | 2.02 | 8 | 9.88 | 5 | 25 | 4.05 | 0.044 |
| Infra auricular fissure | 15 | 0 | 0 | 8 | 9.88 | 7 | 35 | 6.71 | 0.010 |
| Juvenile plantar dermatoses | 11 | 2 | 2.02 | 5 | 6.17 | 4 | 20 | 6.50 | 0.011 |
| Follicular eczema | 7 | 0 | 0 | 2 | 2.47 | 5 | 25 | 4.98 | 0.026 |
| Prurigo nodularis | 4 | 0 | 0 | 3 | 3.7 | 1 | 5 | 1.14 | 0.285 |
Chi-square X2test used and Pvalue <0.05–Significant. AD: Atopic dermatitis, SCORAD Index: SCORing atopic dermatitis index
In our study, symptoms and the course of disease were influenced by certain factors such as itch when sweating, observed in 15% of patients, wool intolerance in 10.50% and seasonal variation with winter exacerbations in 43.50% of patients, while summer flare-ups were seen only in 3.50%. Sendrasoa et al.[16] (2020) reported winter flare-ups in 51.6% of patients, but the study by Parthasarathy et al.[14] (2019) reported a history of winter exacerbation only in 8% of cases. These differences in seasonal variation reported could be attributed to different climatic, ecological and geographical factors which determine varying clinical expressions of the disease in that population.[23] Winter exacerbations were reported more in Northern India compared to Eastern and Southern parts as winters are prolonged and harsh in North India, and there is decreased moisture in the climate, leading to variations in skin moisturisation, thus leading to flare-ups of AD and also direct wearing of woollen clothes triggers pruritus thus influencing the course of disease during winters.[10]
Pruritus is considered a hallmark of AD, which was seen in 100% (200) children with AD. In our study, typical morphology and distribution were seen in 97.50% of cases, where the face was the most commonly affected site amongst infants, 95.08% and flexural involvement was typically more in children, 77.70%. Similar findings were observed by Esenboga et al.[24] (2021) and Kumar et al.[25] (2014), who also reported more facial involvement in infants. Sendrasoa et al.[16] (2020) also reported facial involvement in infants and flexural involvement in children with AD.
The most common type of presentation in infants was acute eczema (63.93%), followed by chronic eczema in 22.95% and subacute in 13.11% of infants while children with AD presented with chronic eczematous lesions observed in 58.27%, followed by subacute in 22.30% and acute papulovesicular erythematous lesions in 19.42%. Acute eczema was reported by Sarkar and Kanwar[10] (2004) in 65.4% of infants, while in children, chronic type predominated in 44.4%. Similarly, Dhar and Kanwar[26] (1998) reported that 52% of infants had acute eczema whereas 47.4% of childhood patients had features of chronic eczema. Chronic/relapsing dermatitis was a common feature in the current study reported in 75% of patients, especially in children >2 years of age and this was in consonance with other studies by Swamy et al.[13] (2019), who reported chronic/relapsing dermatitis in 82% of patients. In this study, the most common minor feature of HRC observed was xerosis seen in 94% of AD patients which could be probably because of decreased ceramides in epidermis along with increased trans-epidermal water loss. Swamy et al. (2019) and Narayan et al. (2019) also reported similar findings.[12,13]
The next common feature of HRC in the present study was hand and foot eczema observed in 41% of children. Narayan et al.[12] (2019) and Parthasarathy et al.[14] (2019) reported comparatively lower frequency as compared to the present study, which could be because this study was done during the COVID-19 pandemic, and frequent use of sanitisers and hand washing might have irritated already compromised epidermal barrier in addition to the use of harsh soaps for bathing and hand washing.
Facial erythema and facial pallor were observed in 40.50% and 6% of children with AD. This occurs because of the abnormal vascular response of superficial dermal blood vessels in AD patients. Narayan et al.[12] (2019) also reported similar findings of facial erythema and facial pallor in 43.6%.
Cutaneous infections were observed in 30.50% of children with AD in the present study. Narayan et al.[12] (2019) and Parthasarathy et al.[14] (2019) reported comparatively lower frequency, while Sehgal et al.[9] (2015) observed a higher frequency of cutaneous infection in their studies.
Dennie Morgan Fold (atopic pleat) was observed in 21.50% of children in our study, and an almost similar finding was reported by Narayan et al. (2019).[12] Other minor features such as periorbital darkening, anterior neck fold, cheilitis, ichthyosis, hyperlinear palms, keratosis pilaris, pityriasis alba and perifollicular accentuation had varied presentations in various studies.[9,12-14] Thus, various studies have shown variable presentation of minor features of HRC, and this could be because of several factors influencing presentation and the course of disease, such as genetic, environmental, climatic, geographical and certain local factors such as clothing, lifestyle and dietary factors.[23,27]
In the present study, amongst atypical features, scalp scaling was the most common feature observed in 17%, followed by retro auricular fissuring in 10%, infra-auricular fissure in 7.5%, eyelid eczema in 7.5%, juvenile plantar dermatoses in 5.5%, follicular eczema in 3.50% and prurigo nodularis in 2% children, respectively. Retro-auricular fissuring, infra-auricular fissuring and scalp scaling were more significantly associated with the severity of AD. Similarly, Narayan et al.[12] (2019) also reported retro-auricular fissures to be significantly associated with disease severity. Infra-auricular fissuring and diffuse scalp scaling are important minor indicators for the diagnosis of AD, which occur as there are high colony counts of pityrosporum in AD patients, which cause subacute dermatitis.[2]
In our study, SCORAD Index grading showed mild severity in 49.50% of children with AD, moderate severity in 40.50% and severe grading in only 10% of cases. Severity grading reported in various Indian and other Asian countries had observed that the majority of cases have mild or mild-to-moderate AD and findings of severity profile in the present study were comparable to previous studies from India and the Asian subcontinent.[11-13,15,25]
Strengths of study
This study highlights not only the most common presenting features and minor features of AD with their frequency of occurrence according to age groups along with severity grades calculated by SCORAD Index but also various atypical clinical features seen in patients of AD. All parameters are evaluated statistically including severity grades and association with atypical features.
Limitations
This was a hospital-based cross-sectional study; hence, the true point prevalence of AD in the community could not be calculated.
CONCLUSION
Various geographical, environmental and local factors, including lifestyle, clothing and dietary habits, influence clinical features, presentation, prevalence and AD severity.
Ethical approval
The ethical approval number is BFUHS/2k20p-TH/15422. The institution name is Baba Farid University of Health Sciences, Faridkot,Punjab. The approval date is 4th February 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Year book of dermatology and syphilology In: Year book of dermatology and syphilology. Chicago: Year Book Publishers; 1933. p. :38-9.
- [Google Scholar]
- Atopic dermatitis in infants and children in India. Indian J Dermatol Venereol Leprol. 2010;76:504-13.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233-46.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of childhood atopic dermatitis and economic burden of illness in Asia Pacific countries. Ann Nutr Metab. 2015;66(Suppl 1):18-24.
- [CrossRef] [PubMed] [Google Scholar]
- Sample size determination In: Research methodology with statistics for health and social sciences. Vol 1. Ilorin: Nathadex Publishers; 2003. p. :117-8.
- [Google Scholar]
- Japanese guidelines for atopic dermatitis 2017. Allergol Int. 2017;66:230-47.
- [CrossRef] [PubMed] [Google Scholar]
- Practical issues on interpretation of scoring atopic dermatitis: The SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007;157:645-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of atopic dermatitis among children in Jaipur. Sch J App Med Sci. 2017;5:1875-8.
- [Google Scholar]
- Atopic dermatitis: A cross-sectional (descriptive) study of 100 cases. Indian J Dermatol. 2015;60:519.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-epidemiological profile and factors affecting severity of atopic dermatitis in North Indian Children. Indian J Dermatol. 2004;49:117.
- [Google Scholar]
- Epidemiology and clinical pattern of atopic dermatitis in 100 children seen in a city hospital. Indian J Dermatol. 2002;47:202-4.
- [Google Scholar]
- Clinicoepidemiologic profile and the cutaneous and nasal colonisation with methicillin-resistant Staphylococcus aureus in children with atopic dermatitis from North India. Indian Dermatol Online J. 2019;10:406-12.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological profile and clinical pattern of atopic dermatitis in South Indian teaching hospital. Indian J Clin Exp Dermatol. 2019;5:146-53.
- [CrossRef] [Google Scholar]
- A study to estimate the frequency of Hanifin and Rajka's minor criteria in children for diagnosis of atopic dermatitis in a tertiary care centre in South India. Indian J Paediatr Dermatol. 2019;21:31-5.
- [CrossRef] [Google Scholar]
- The clinico-epidemiological profile of atopic dermatitis in residential schoolchildren: A study from South Chhattisgarh, India. Indian J Paediatr Dermatol. 2017;18:281-5.
- [CrossRef] [Google Scholar]
- Epidemiology and associated factors of atopic dermatitis in Malagasy children. Allergy Asthma Clin Immunol. 2020;16:4.
- [CrossRef] [PubMed] [Google Scholar]
- Scoring atopic dermatitis and six sign atopic dermatitis: Comparison of prognostic and predictive value in atopic dermatitis. Indian J Paediatr Dermatol. 2013;14:13-8.
- [CrossRef] [Google Scholar]
- Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13:15-26.
- [CrossRef] [PubMed] [Google Scholar]
- Personal and family history of atopy in children with atopic dermatitis in north India. Indian J Dermatol. 1997;42:9.
- [Google Scholar]
- Atopic dermatitis in children: A clinico epidemiological study and the role of dietary restrictions in disease severity. Int J Res Dermatol. 2017;3:168-74.
- [CrossRef] [Google Scholar]
- Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980;92:44-7.
- [CrossRef] [Google Scholar]
- Clinico-immunological profile and their correlation with severity of atopic dermatitis in Eastern Indian children. J Nat Sci Biol Med. 2014;5:95-100.
- [CrossRef] [PubMed] [Google Scholar]
- The International study of asthma and allergies in childhood (ISAAC) phase three: A global synthesis. Allergol Immunopathol (Madr). 2013;41:73-85.
- [CrossRef] [PubMed] [Google Scholar]
- Infantile atopic dermatitis: Serum vitamin D, zinc and TARC levels and their relationship with disease phenotype and severity. Allergol Immunopathol (Madr). 2021;49:162-8.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological pattern of psoriasis, vitiligo and atopic dermatitis in India: Hospital-based point prevalence. Indian Dermatol Online J. 2014;5:S6-8.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and clinical pattern of atopic dermatitis in a North Indian pediatric population. Pediatr Dermatol. 1998;15:347-51.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of atopic dermatitis: A review. Allergy Asthma Proc. 2012;33:227-34.
- [CrossRef] [PubMed] [Google Scholar]






