Translate this page into:
Pulmonary function tests in children with beta-thalassemia major
*Corresponding author: Vinod H. Ratageri, Department of Pediatrics, Karnataka Institute of Medical Sciences, Hubli, Karnataka, India. ratageri@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Harsoor J, Ratageri VH, Shilpa C, Illalu S, Wari P. Pulmonary function tests in children with beta-thalassemia major. Karnataka Paediatr J 2020;35(1):52-6.
Abstract
Objectives:
The objective of the study was to study the pattern of lung functions in thalassemia major children and correlation of pulmonary function tests (PFTs) with serum ferritin.
Materials and Methods:
A hospital-based cross-sectional descriptive study done from January 2017 to December 2017. Inclusion criteria: Children with confirmed diagnosis of beta-thalassemia major in the age group of 5–15 years were included in the study. Exclusion criteria: Already diagnosed cases of pulmonary dysfunctions, CHD and RHD were excluded from the study. All enrolled children underwent a detailed clinical history, physical examination and blood sample were sent for Hb and serum ferritin before blood transfusion (BT). PFT was done within 24 h of BT using spirometer (Helios-401). Statistical analysis was done using SPSS (Version22).
Results:
Forty-five children enrolled in the study and majority of them were <10 years (37 children) with M:F ratio 1.6:1. The pulmonary dysfunction was present in 35 (77.8%), but none of them had respiratory symptoms. The pulmonary dysfunction observed was restrictive 31 (88.5%), obstructive 2 (5.7%), and combined 2 (5.7%). A reduced forced vital capacity (FVC) % in 33 (73.3%), a reduced forced expiratory volume in the 1st second (FEV1%) in 25 (55.5%), a normal FEV1/FVC in 41 (91.2%), and a reduced FEF 25–75% in 23 (51.1%) children were observed. Risk factors such as, age, height, and duration of chelation (>5 years) were significantly associated with pulmonary dysfunction (P < 0.05). There was no correlation between serum ferritin levels and PFT. However, PFT values were found to be decreased in patients with a high serum ferritin (>2500 ng/ml), but these differences were statistically not significant.
Conclusion:
Abnormal patterns of lung function were common (restrictive type, predominant), even though none of these children had any respiratory symptoms.
Keywords
Pulmonary function tests
Serum ferritin
Thalassemia
Children
INTRODUCTION
Thalassemia is an autosomal recessive disorder and is the most common monogenic disorder worldwide. About 10% of the total world thalassemics are born in India every year.[1] In India, the prevalence of beta-thalassemia is 1–17% and carrier frequency is 3–4%.[2] Over the past three decades, regular blood transfusions (BTs) and iron chelation have dramatically improved the quality of life and thalassemia is transformed from a rapidly fatal disease to a chronic disease and is compatible with prolonged life. Regular BT causes generalized iron overloading in organs such as heart, liver, and pancreas. Lung impairment in thalassemia is also noted. Although, it does not produce any symptoms and is not the most significant clinical manifestation of thalassemia.
Most studies found that restrictive dysfunction is the predominant pattern of lung function abnormality,[3-11] although some others found obstructive lung dysfunction[12-14] and combined[15] pattern also. The precise causes and pattern of pulmonary dysfunction in thalassemia has not yet been established. Most of the studies have tried to correlate serum ferritin levels with pulmonary function abnormalities, but the results are conflicting.[3-6,9,12,16] However, lung dysfunction has never been adequately focused upon and remains to be one of the least understood complications, hence, we propose to study the pattern of lung functions in thalassemia major children and correlation of lung function test with serum ferritin.
MATERIALS AND METHODS
Prospective hospital-based cross-sectional study, during January 1, 2017, to December 31, 2017, in children aged between 5 and 15 years with beta-thalassemia major admitted for periodic BT in pediatric ward, at Karnataka Institute of Medical Sciences, Hubli.
Inclusion criteria
Children with confirmed diagnosis of beta-thalassemia major in the age group of 5–15 years were included in the study.
Exclusion criteria
(i) Thalassemia children who were already diagnosed cases of pulmonary dysfunctions (i.e., asthma, bronchiectasis, and other chronic lung diseases) and (ii) children with congenital heart disease/rheumatic heart disease were excluded from the study.
Sample size
Based on the previous literature, data indicate that the prevalence of abnormal pulmonary function test (PFT) in children with beta-thalassemia admitted to pediatric ward is 86–95%. To estimate the prevalence of abnormal PFT in children with beta-thalassemia within 6 percentage points of the true value of 90% with 80% confidence, we require a minimum of 41 beta-thalassemia major children to be studied.
Methodology
Ethical clearance was obtained from the Institutional Ethics Committee. Children who fulfill the inclusion/exclusion criteria for the study were selected. Informed and written consent was obtained from parents of all cases. All enrolled children were taken detailed clinical history including age at first BT, number of BT, duration of iron chelation therapy, and general physical examination findings which were recorded on a predesigned pro forma. Before BT, for all enrolled children, one blood sample was sent for serum ferritin level and another sample for pre-transfusion Hb. Serum ferritin level was measured by electrochemiluminescence technique using Cobas 6000 analyzer. PFT was done using spirometer (RMS Helios-401), within 24 h of BT. Standard procedure for PFT was carried out to all enrolled children and then checked test for acceptability and reproducibility.[17] The following parameters were recorded in the spirometry – forced vital capacity (FVC), forced expiratory volume in the 1st second (FEV1), ratio of FEV1/FVC, peak expiratory flow rate (PEFR), and forced expiratory flow between 25 and 75% vital capacity (FEF 25–75%). Interpretation of PFT was done according to the recommended guidelines by asthma training module.[17]
Statistical analysis
Data were entered into Microsoft Excel data sheet and were analyzed using SPSS 22 version software (IBM SPSS Statistics, Somers NY, USA) that was used to analyze data. Chi-square test was used as test of significance for qualitative data. Continuous data were represented as mean and SD. Analysis of variance was the test of significance to identify the mean difference between more than 2 groups for quantitative data. Independent t-test was the test of significance to identify the mean difference between less than 2 groups for quantitative data. Pearson correlation was done to find the correlation between two quantitative variables.
RESULTS
Total number of children included in the study was 45. Thirty-seven (82.2%) children were <10 years and 8 children (17.8%) were more than or equal to 10 years, with mean age 7.78 ± 2.4 and male:female ratio 1.6:1. All children (100%) presented with easy fatigability and progressive pallor. Only 2 (4.4%) children had presented with cough secondary to URTI. None of the children presented with rapid breathing, chest in drawing, and chest pain. On per abdomen examination, all children had hepatomegaly (100%) and 34 children (75.5%) had splenomegaly, remaining 11 (24.5%) children were splenectomized. [Table 1] shows baseline character of enrolled children.
| Baseline characteristics | Mean±SD | |
|---|---|---|
| Anthropometry | ||
| Weight (kg) | 17.8±4.8 | |
| Height (cm) | 111.8±13.7 | |
| BMI | 14.0±1.4 | |
| No. of blood transfusion | ||
| <50 (n=9) | 36.4±6.9 | |
| 50–100 (n=19) | 78.8±10.6 | |
| >100 (n=3) | 106.3±3.0 | |
| Duration of chelation (years) | ||
| Total (n=45) | 4.2±1.4 | |
| <5 years | n=25 (55.5%) | |
| >5 years | n=20 (44.4%) | |
| Pre-transfusion HB (g/dl) | 5.7±1 | |
| Post-transfusion HB(g/dl) | 9.7±0.8 | |
| Serum ferritin (ng/ml) | ||
| Total | 3085.4±2184.7 | |
| <2500 (n=23) | 1545±461.5 | |
| >2500 (n=22) | 4695.6±2112.6 |
Total number of children with abnormal PFTs was 35 (77.8%). Among 35 children with pulmonary dysfunction, 31 (88.5%) children had restrictive pattern, 2 (5.71%) children had obstructive pattern, and 2 (5.71%) children had combined pattern.
[Table 2] shows mean values of various parameters of PFT. Twenty-five (55.5%) children had reduced FEV1 and 33 (73.3%) had reduced FVC. However, 41 (91.1%) had normal FEV1/FVC%, whereas PEFR and FEF 25–75% were reduced in 22 (51.1%) children each.
| PFT parameters | Mean±SD | Number of cases (n=45) | |
|---|---|---|---|
| Normal (%) | Decreased (%) | ||
| FEV1% | 81.7±40.6 | 20 (44.4) | 25 (55.5) |
| FVC% | 68.9±20.6 | 12 (26.7) | 33 (73.3) |
| FEV1/FVC% | 121.4±39.8 | 41 (91.1) | 4 (8.9) |
| PEFR | 99.3±49.9 | 22 (48.9) | 23 (51.1) |
| PEF 25–75% | 82.4±31.6 | 22 (48.9) | 23 (51.1) |
[Tables 3 and 4] show risk factors for pulmonary dysfunction. There was a significant negative correlation between age and height with respect to FVC%, FEV1%, and PEFR, but there was no significant correlation between BMI and PFT parameters (P > 0.05). [Figure 1] depicts scattered plot showing comparison between height and FVC% predicted (negative correlation). There was a significant difference in mean FVC%, FEV1%, and PEFR with respect to the duration of chelation (P < 0.05%). Mean FVC%, FEV1%, and PEFR were significantly reduced among those with duration of chelation (>5 years).
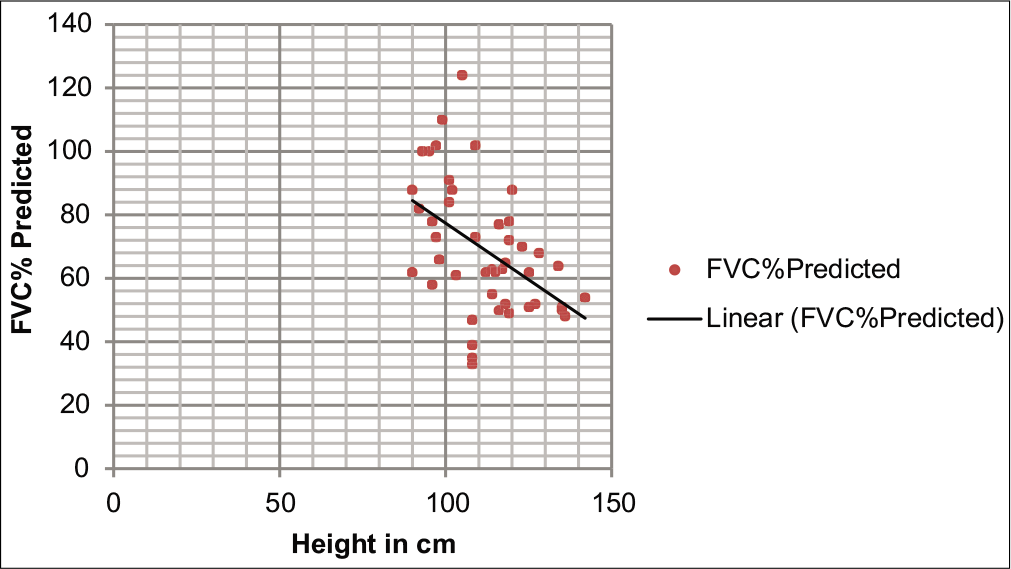
- Scattered plot showing comparison between height and forced vital capacity % predicted.
| FVC% | FEV1% | FEV1/FVC% | PEFR | |
|---|---|---|---|---|
| Age | ||||
| Pearson correlation | ‒0.435** | ‒0.324* | ‒0.081 | ‒0.507** |
| P-value | 0.003 | 0.03* | 0.595 | <0.001* |
| Height | ||||
| Pearson correlation | ‒0.474** | ‒0.414** | ‒0.195 | ‒0.574** |
| P-value | 0.001* | 0.005* | 0.199 | <0.001* |
| BMI | ||||
| Pearson correlation | 0.127 | 0.148 | 0.086 | ‒0.092 |
| P-value | 0.405 | 0.332 | 0.573 | 0.546 |
| PFT | Duration of chelation (year) | P-value# | |
|---|---|---|---|
| Mean with SD | |||
| <5 years (n=25) | >5 years (n=20) | ||
| FVC% | 75.08±22.46 | 61.25±15.14 | 0.023* |
| FEV1% | 92.68±49.24 | 68.05±19.93 | 0.042* |
| FEV1/FVC% | 124.64±52.63 | 116.74±11.32 | 0.514 |
| PEFR | 117.84±58.86 | 75.46±18.77 | 0.003* |
| PEF 25–75% | 83.92 | 80.45 | 0.719 |
Mean serum ferritin levels in children with normal PFT, restrictive pattern, obstructive pattern, and combined pattern were 2868.70 ± 2985.58, 3267.93 ± 1995.89, 2792.40 ± 2096.01, and 1633.15 ± 32.03, respectively. There was no significant difference in mean values of PFT parameters, age, BMI, and pre-transfusion Hb with respect to serum ferritin levels; however, FVC and FEV1, PEFR, and PEF 25–75% values were found to be decreased in children with a high ferritin level (>2500 ng/ml) as compared with children with a low ferritin level (<2500 ng/ml), but these differences were statistically not significant [Tables 5 and 6].
| Serum ferritin | FVC% | FEV1% | FEV1/FVC% | PEFR | PEF 25–75% | ||
|---|---|---|---|---|---|---|---|
| Serum ferritin | Pearson correlation | 1 | 0.036 | 0.026 | 0.022 | ‒0.075 | 0.128 |
| P-value | 0.814 | 0.866 | 0.885 | 0.626 | 0.402 |
| Serum ferritin | P-value# | ||||||
|---|---|---|---|---|---|---|---|
| <2500 ng/dl (n=23) | >2500 ng/dl (n=22) | ||||||
| Mean | SD | Median | Mean | SD | Median | ||
| FVC% | 71.04 | 22.81 | 65.00 | 66.73 | 18.16 | 62.50 | 0.488 |
| FEV1% | 87.17 | 46.31 | 80.00 | 76.05 | 33.73 | 70.00 | 0.364 |
| FEV1/FVC% | 122.60 | 48.09 | 123.00 | 119.59 | 29.77 | 122.00 | 0.803 |
| PEFR | 102.35 | 51.55 | 76.00 | 95.50 | 49.19 | 83.00 | 0.651 |
| PEF 25–75% | 77.30 | 30.34 | 78.00 | 87.68 | 32.74 | 82.00 | 0.276 |
| Age | 7.22 | 2.15 | 7.00 | 8.36 | 2.59 | 8.50 | 0.113 |
| BMI | 13.90 | 1.55 | 13.60 | 14.06 | 1.29 | 13.88 | 0.711 |
| PT-Hb | 5.63 | 1.17 | 5.70 | 5.86 | 0.79 | 6.00 | 0.449 |
DISCUSSION
Lung dysfunction is among the least studied complication in children with beta-thalassemia major, probably due to the lack of pulmonary symptoms presenting compared to cardiomyopathy or endocrine complication.[16] We observed that 35 (77.8%) children had abnormal PFT. The pulmonary functional abnormality of frequency was restrictive 31 (88.5%), obstructive 4 (5.7%), and combined 4 (5.7%) pattern. Impairment in respiratory function among thalassemia children has been reported in the range of 29–86%.[7-9]
Restrictive lung function abnormality was observed as major pulmonary abnormality in our study. Several other studies[3-11] have shown similar restrictive pattern as major pulmonary dysfunction. However, none of them found exact etiopathogenesis. We analyzed various risk factors for abnormal lung function including age, height, BMI, number of BT, duration of chelation therapy, organomegaly, and serum ferritin. Of these, age, height, and duration of chelation therapy (>5 years) were significantly associated with pulmonary dysfunction (FVC%, FEV1, and PEFR). Our findings were consistent with the previous literature, as reported by others.[4,8] The severity of restrictive abnormalities of thalassemic children was found to increase with age. In many other studies, different etiopathogenetic mechanisms for the development of restrictive lung dysfunction were reported such as multiple BT,[18] hypoxia,[18] iron overload,[4,5,9] drug like desferrioximine,[8,18] and genetic structure.[18]
Four children (5.7%) had obstructive type of lung dysfunction. Similar results were found in other studies.[6,9] However, Gulhan et al.[12] found more number of obstructive dysfunction (46.2%). We also observed the combined pattern in 4 children (5.7%). Similar finding was also observed in Said[15] (3.1%). The probable reason for small number of cases could be due to limitations in screening.
The mean values of various parameters of PFTs were reduced. A reduced FVC% 33 (73.3%), a reduced FEV1 25 (55.5%), a normal FEV1/FVC ratio 41 (91.2%), and a reduced FEF 25–75% 23 (51.1%) were observed. In our study, pulmonary function abnormality may be partially explained by insufficient anatomic and functional development of lung during early infancy,[4] as we observed age and height negatively correlated with various parameters of PFT. The other reasons could be iron overload as serum ferritin (>2500 ng/ml) had significantly associated with pulmonary dysfunction.
Although the measurement of serum ferritin is not the best quantitative estimate of body iron stores, thalassemic patients with a serum ferritin concentration of ≥3000 ng/dL have been reported to have a high probability of lung injury.[19] Levels >2500 ng/dL have been reported to be associated with a 4-fold higher risk of death.[3] In the present study, no correlation was found between serum ferritin levels and PFT; however, we observed, FVC and FEV1, PEFR, and FEF 25–75% values were found to be decreased in patients with a high ferritin level (>2500 ng/dL) as compared with children with a low ferritin level (<2500 ng/dL), but these differences were statistically not significant. Probably, as our numbers are less. A complex mechanism in addition to iron overload has been proposed to play important role in the development of lung dysfunction.[13]
In the present study, age and height were inversely correlated with FVC%, FEV1%, and PEFR. There was a significant negative correlation between height and FVC%, FEV1%, and PEFR, that is, with increase in height, there was a decrease in FVC%, FEV1%, and PEFR. Probable cause could be the growth retardation which was observed in thalassemic children and the predictive lung volume, which was derived from age and height, might not be an accurate value, even if the effect of thalassemia on lung growth could be different from one on general growth.[18,20]
Keens et al.[21] suggested that good compliance to chelation therapy is crucial to prevent complications in these children. In our study, FVC%, FEV1%, and PEFR were significantly reduced among those with duration of chelation (>5 years). Even though all children had received chelation therapy, still we found significant reduction in PFT parameters; this could be probably due to inadequate dosage or non-compliance of the drug. Similar results found Said study.[15]
CONCLUSION
Majority of our thalassemia children (78%) had abnormal patterns of lung function even though none of these children had any respiratory symptoms. We suggest, all children with thalassemia on regular BT should undergo PFT annually to prevent the sequelae.
Declaration of patient consent
The Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Molecular genetic analyses of beta thalassemia in South India reveal rare mutations in the beta globin gene. J Hum Genet. 2004;49:408-13.
- [CrossRef] [PubMed] [Google Scholar]
- Beta thalassemia in India: Current status and the challenges ahead. Int J Pharm Pharm Sci. 2014;6:28-33.
- [Google Scholar]
- Pulmonary functions in children with thalassemia major. J Pediatr Hematol Oncol. 2015;37:605-10.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary function tests in children with beta-thalassemia major. Chron Respir Dis. 2007;4:19-22.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary dysfunction in children with beta thalassemia major in relation with iron overload. Asian J Med Sci. 2015;6:47-50.
- [CrossRef] [Google Scholar]
- Study of pulmonary function tests in thalassemic children. J Pediatr Hematol Oncol. 2007;29:151-5.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary function tests in beta thalassemia. Indian J Pediatr. 2001;68:239-42.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary function abnormalities in thalassemia major and the role of iron overload. Am J Respir Crit Care Med. 1994;149:1570-4.
- [CrossRef] [PubMed] [Google Scholar]
- Relation of ferritin levels to pulmonary function in patients with thalassemia major and the acute effects of transfusion. Eur J Haematol. 2000;64:396-400.
- [CrossRef] [PubMed] [Google Scholar]
- Low lung capacity and hypoxemia in children with thalassemia major. Am Rev Respir Dis. 1980;121:639-46.
- [Google Scholar]
- Lungs in thalassaemia major patients receiving regular transfusion. Eur Respir J. 1996;9:1389-94.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of blood transfusion on cytokine profile and pulmonary function in patients with thalassemia major. Clin Respir J. 2016;10:153-62.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of transfusion on pulmonary function in patients with thalassemia major. Pediatr Pulmonol. 1994;18:139-43.
- [CrossRef] [PubMed] [Google Scholar]
- Betathalassemia major: Thin-section CT features and correlation with pulmonary function and iron overload. Radiology. 2003;229:507-12.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of pulmonary functions of thalassemic and of healthy children. Paediatr Indones. 2005;45:1-6.
- [CrossRef] [Google Scholar]
- Pulmonary function test in transfusion-dependent β-thalassemia major patients: A pilot study. Pediatr Hematol Oncol. 2011;28:329-33.
- [CrossRef] [PubMed] [Google Scholar]
- IAP National Guidelines for the Management of Childhood Asthma India: Asthma Training Module; 2016.
- [Google Scholar]
- Evidence of a restrictive spirometric pattern in older thalassemic patients. Respiration. 2001;68:273-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary dysfunction in transfusion-dependent patients with thalassemia major. Am J Respir Crit Care Med. 2003;168:180-4.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary function abnormalities in homozygous beta-thalassemia. J Pediatr. 1986;109:452-5.
- [CrossRef] [Google Scholar]
- Pulmonary function abnormalities in thalassemia patients on a hypertransfusion program. Pediatrics. 1980;65:1013-7.
- [Google Scholar]






